Key Points
Motility of resting CLL cells requires chemokine-mediated RhoA activation but is independent of Tiam1/Rac signals.
Tiam1/Rac signals are indispensible for CLL cell proliferation and chemoresistance.
Abstract
Signals from the tumor microenvironment promote the migration, survival, and proliferation of chronic lymphocytic leukemia (CLL) cells. Rho GTPases control various signaling pathways downstream of microenvironmental cues. Here, we analyze the function of Rac1 in the motility and proliferation of CLL cells. We found decreased transcription of the Rac guanine nucleotide exchange factors Tiam1 and Vav1 in unstimulated peripheral blood CLL cells with almost complete loss of Tiam1 but increased transcription of the potential Rac antagonist RhoH. Consistently, stimulation of CLL cells with the chemokine CXCL12 induced RhoA but not Rac1 activation, whereas chemokine-induced CLL cell motility was Rac1-independent. Coculture of CLL cells with activated T cells induced their activation and subsequent proliferation. Here, Tiam1 expression was induced in the malignant cells in line with increased Ki-67 and c-Myc expression. Rac1 or Tiam1 knockdown using siRNA or treatment with the Tiam1/Rac inhibitor NSC-23766 attenuated c-Myc transcription. Furthermore, treatment of CLL cells with NSC-23766 reduced their proliferation. Rac inhibition also antagonized the chemoresistance of activated CLL cells toward fludarabine. Collectively, our data suggest a dynamic regulation of Rac1 function in the CLL microenvironment. Rac inhibition could be of clinical use by selectively interfering with CLL cell proliferation and chemoresistance.
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent leukemia in the Western world and is still considered incurable. It is increasingly recognized that microenvironmental interactions of CLL cells within bone marrow and lymph nodes essentially affect CLL pathophysiology, and that disrupting these interactions bears a high therapeutic potential.1 Although almost all CLL cells in the peripheral blood are cell cycle-arrested, aggregates of proliferating CLL cells are found in the lymph nodes and, to a lesser extent, in the bone marrow.2 Here, CLL cell proliferation appears to be primarily induced by their crosstalk with CD4+ T cells.3,4 Other accessory cells, and in particular stromal cells, further support the activation and survival of malignant cells.5-8
The correct localization of CLL cells adjacent to favorable microenvironmental signals provided by bystander cells requires the integration of adhesive and migratory signals into cytoskeletal responses. In addition, coordinated and dynamically regulated changes in the cytoskeleton are mandatory for tumor cell proliferation. Rho GTPases play a major role in the organization of the actin cytoskeleton, as well as in the control of gene expression programs involved in proliferation and survival.9,10 In hematopoietic stem and progenitor cells, Rho GTPases have been described as key mediators of microenvironmental signals downstream of the chemokine receptor CXCR4 and β1 integrins, which are also known to orchestrate CLL cell migration and survival.11-13 Rho proteins mainly function as guanine nucleotide-dependent molecular binary switches alternating between an inactive GDP-bound and an active GTP-bound conformational state, which is induced by guanine nucleotide exchange factors (GEFs). The best-studied members of the Rho GTPase family in hematopoietic cells are RhoA and Rac1.11,14
In CLL, RhoA has been suggested as a contributor to the induction of chemokine-induced integrin activation and migration.15,16 The role of Rac activity and usage in CLL is still elusive. We investigated RhoA and Rac1 function in CLL cell motility and proliferation. We found that the motility of resting CLL cells was dependent on the RhoA effector molecule Rho-associated coiled-coil forming protein kinase (ROCK) but was independent of active Rac1. This independency was accompanied by a severe downregulation of the major Rac GEF T lymphoma invasion and metastasis 1 (Tiam1) in resting CLL cells. However, once activated, CLL cells restored Tiam1 expression, and CLL cell proliferation involved the Rac1/Tiam1 axis, suggesting a dichotomy of Tiam1/Rac usage in resting vs proliferating CLL cells.
Materials and methods
Materials
Fluorochrome-labeled monoclonal antibodies were purchased from Becton-Dickinson, Dako-Cytomation, Beckman Coulter, and eBioscience. Chemokines were supplied by R&D Systems. All other reagents and chemicals were purchased from Sigma-Aldrich unless otherwise indicated.
Patient samples
Blood samples from patients with CLL at the Third Medical Department Salzburg were obtained on written informed consent and under the approval of the Salzburg Ethics Committee, Salzburg, Austria (415-E/1287/4-2011, 415-E/1287/8-2011). This study was conducted in accordance with the Declaration of Helsinki. Analyzed patients were chemonaive or had received no therapy for the last 6 months. For patient characteristics, see supplemental Table 1, available on the Blood Web site. Peripheral blood mononuclear cells from patients with CLL or healthy donors were isolated by density gradient centrifugation and either were used freshly or were viably frozen. Thawed cells were cultured in RPMI-1640 containing 10% fetal calf serum, glutamine, and antibiotics. Negative or positive selection of healthy B lymphocytes or CLL cells was performed using the EasySep system from Stem Cell Technologies or the anti-CD19 antibody-based MACS separation system (Miltenyi Biotec). CLL cell purity was higher than 95%.
Real-time polymerase chain reaction
Total RNA from CD19-sorted B lymphocytes or CLL cells was isolated using the QIAamp RNA Blood Mini Kit (Qiagen). Reverse transcription of RNA was performed using the i-script cDNA Synthesis Kit (Bio-Rad). Quantitative real-time polymerase chain reaction (PCR) using Taqman Gene Expression Assays was performed using the 7500 Real-Time PCR System (Applied Biosystems). Results were quantified using the ΔΔCT method, with 18S rRNA as a control (for ΔCT and CT values, see supplemental Table 2).
Cell motility
µ-slides (ibidi) were coated with 100 µg/mL collagen IV (Stem Cell Technologies) overnight at 4°C, followed, where indicated, by 1 hour coating with 100 ng/mL CXCL12, washing, and blocking with 2% human serum albumin. For inhibition experiments, CLL cells were pretreated with 50 µM NSC-23766, 10 µM Y-27632, 100 ng/mL Pertussis toxin, 10 µM src inhibitor PP2, or solvent for 30 minutes. The chambers were placed on a heated (37°C) stage of an inverted phase-contrast microscope (Olympus IX81), and at least 60 cells per field were recorded by time-lapse videomicroscopy, using a 20-fold magnification. Motile cells were determined by manual tracking of single cells.13,17 Cells moving more than 5 cell diameters during the observation time of 1 hour were considered motile. Representative differential interference contrast (DIC) images were taken with ×96 magnification.
Proliferation
CLL cell proliferation was induced as described.18 PBMCs from patients with CLL were cultured with fibroblasts (NIH/3T3 cell line) with or without 10 µL/mL of activating anti-CD3/CD28 microbeads (Life Technologies) for 5 days, and cell proliferation of CD5+/CD19+ cells was assessed by an intracellular staining, using an antibody against Ki-67 or isotype control, and analyzed by flow cytometry. Alternatively, CLL cells were activated via their coculture with CD40L-transfected fibroblasts, as described.18
RNA interference
PBMCs from patients with CLL were resuspended in Opti-MEM reduced serum cell culture medium (Life Technologies) and transfected with 100 pmol small interfering RNA (siRNA), using the Amaxa nucleofector apparatus and the C-05 program according to the manufacturer's instructions (Lonza AG). After transfection with nontargeting-siRNA, Tiam1-siRNA, or Rac1-siRNA (Ambion), cells were activated using 10 µL/mL anti-CD3/C28 beads for 24 hours. RNA was isolated from anti-CD19 MACS-sorted CLL cells and further used for real-time PCR.
Cell viability assay
CD5 and CD19 expression was assessed using fluorochrome-labeled antibodies, and cell viability was cytometrically analyzed using annexin V-fluorescein isothiocyanate and 7-aminoactinomycin D and a FC-500 flow cytometer (Beckman Coulter). Viable cells were defined as annexin V/7-AAD double-negative.
RhoA and Rac1 activity assay
The activity of RhoA and Rac1 was measured using the RhoA and Rac1 G-LISA assays (Cytoskeleton). CLL cells were harvested in ice-cold phosphate-buffered saline after stimulation and lysed immediately. Equal protein loading was confirmed using a Bradford assay. Activated Rho or Rac was captured in 96-well plates using its substrates Rhotekin or Pak for RhoA and Rac1, respectively. After washing away unbound nonactivated GTPase, activated RhoA or Rac1 was detected using specific horseradish peroxidase-conjugated antibodies. Chemiluminescence was detected by a DTX880 multiplate reader (Beckman Coulter).
Western blotting
Cell lysates of CD19+-selected CLL cells were separated using 10% sodium dodecyl sulfate/polyacrylamide gel electrophoresis. After transferring the proteins onto a polyvinylidene fluoride membrane, immune detection of specific proteins was performed using appropriate antibodies (1:1000 to 1:2000) and enhanced chemiluminescence detection. Images were analyzed using the ImageJ software, V1.45s.
Statistical analyses
Statistical analyses were performed using the GraphPad Prism software. All data were tested for normal distribution. Comparisons of normally distributed data from 2 groups were done with t tests (paired or unpaired Student t tests, respectively), and nonparametric data were compared using Wilcoxon signed rank test (paired analysis) or Mann-Whitney test (unpaired analysis). For comparing 3 or more groups, 1-way analysis of variance and respective nonparametric tests and the corresponding post hoc tests were used. Differences were considered statistically significant when P < .05, with values at P < .05 marked as *, <.01 as **, and <.001 as ***. Nonsignificant differences are marked as n.s.
Results
CLL cells display a specific motility mode that is independent of Rac/Tiam signals
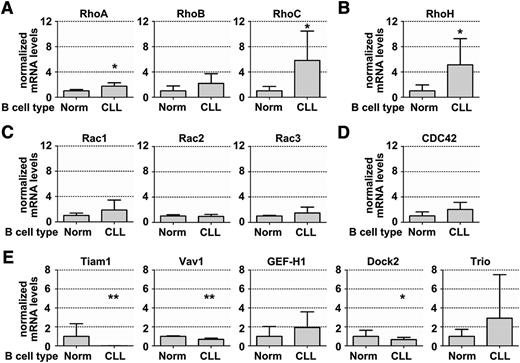
To get a first insight into the expression of different members of the Rho GTPase family in CLL, we profiled the transcript levels of common lymphocyte-relevant Rho GTPases and GEFs by real-time PCR. Comparing CLL cells with healthy B cells, we found significantly higher messenger RNA (mRNA) levels of RhoA, RhoC, and RhoH, but lower expression of the GEF Vav1 (Figure 1A-E). Notably, the GEF Tiam1 was downregulated to almost a complete loss in CLL cells compared with normal B cells (Figure 1E), irrespective of the clinical risk profile of the CLL samples (supplemental Figure 1).
Gene expression profiling of GTPases and GEFs in CLL and healthy B cells revealed loss of the Rac activator Tiam1 in CLL. (A-E) Relative gene expression profile of GTPase family members in sorted peripheral blood-derived CLL cells compared with normal B lymphocytes. Real-time PCR was performed using TaqMan primers and probes, as described in Materials and methods. (A) RhoA mRNA expression in healthy B (n = 3) and CLL (n = 18) cells, RhoB in healthy B (n = 7) and CLL (n = 8) cells, and RhoC in healthy B (n = 7) and CLL (n = 8) cells. (B) RhoH expression in healthy B (n = 7) and CLL (n = 8) cells. (C) Rac1, Rac2, and Rac3 in healthy B (n = 3) and CLL (n = 18) cells. (D) CDC42 expression in healthy B (n = 3) and CLL (n = 18) cells. (E) Tiam1 expression in healthy B (n = 7) and CLL (n = 8) cells and Vav1 and Dock2 expression in healthy B (n = 3) and CLL (n = 18) cells. All data represent means ± standard deviation (SD).
Gene expression profiling of GTPases and GEFs in CLL and healthy B cells revealed loss of the Rac activator Tiam1 in CLL. (A-E) Relative gene expression profile of GTPase family members in sorted peripheral blood-derived CLL cells compared with normal B lymphocytes. Real-time PCR was performed using TaqMan primers and probes, as described in Materials and methods. (A) RhoA mRNA expression in healthy B (n = 3) and CLL (n = 18) cells, RhoB in healthy B (n = 7) and CLL (n = 8) cells, and RhoC in healthy B (n = 7) and CLL (n = 8) cells. (B) RhoH expression in healthy B (n = 7) and CLL (n = 8) cells. (C) Rac1, Rac2, and Rac3 in healthy B (n = 3) and CLL (n = 18) cells. (D) CDC42 expression in healthy B (n = 3) and CLL (n = 18) cells. (E) Tiam1 expression in healthy B (n = 7) and CLL (n = 8) cells and Vav1 and Dock2 expression in healthy B (n = 3) and CLL (n = 18) cells. All data represent means ± standard deviation (SD).
Directed lymphocyte migration is controlled by the dynamic activation of RhoA and Rac1.19 Tiam1 has been identified as a Rac-specific GEF20,21 that organizes T-cell polarization, migration, and homing.22 Moreover, RhoH is a hematopoiesis-specific Rho GTPase23 with constitutive activity and potential Rac-inhibitory function,24 which has recently been identified as being involved in tumor development and CLL cell–microenvironment interactions.25,26 Hypothesizing that the lack of Tiam1 combined with the overexpression of the potential Rac antagonist RhoH may affect Rac activation and, consequently, cell motility in CLL, we investigated chemokine-induced Rac1 activation compared with RhoA activation. Indeed, using quantitative G-LISA assays,27,28 we observed CXCL12-induced RhoA in CLL cells that were interacting with a CXCL12-collagen substrate, inducing their motility (Figure 2A, left). We also detected CXCL12-induced RhoA activation in healthy B cells (supplemental Figure 2A). However, we did not detect Rac1 activation in CLL cells in contact with the CXCL12-collagen substrate (Figure 2A, right). Stimulation of CLL cells with soluble CXCL12 did not induce Rac1 activation either (data not shown). As an additional technical control, we used a classical Rac1 activation pull-down assay. CXCL12-induced Rac1 activation of peripheral blood mononuclear cells from healthy donors was clearly detectable in this system, whereas a CLL-derived Rac activation signal could not be observed (supplemental Figure 2B). A second chemokine, the CCR7 ligand CCL19, also failed to provoke Rac1 activation in CLL cells (Figure 2B).
CXCL12-mediated CLL cell motility is dependent on a functional Rho/ROCK signal, whereas Rac is dispensable. (A) CLL cells were cultured on immobilized CXCL12 or collagen (COLL)/CXCL12, and activation of Rac1 (left) and RhoA (right) was analyzed (n = 4). (B) Rac1 activation in CCL19-treated CLL cells (n = 4). (C) Western blot analysis of phosphorylation of ezrin/radixin/moesin proteins and Vav1 from CLL cells cultured on immobilized CXCL12 or COLL/CXCL12. A representative blot out of 3 independent experiments is shown. (D) CLL cells were subjected to COLL with or without CXCL12, and cell motility was assessed using time-lapse videomicroscopy (n = 6) (left); a representative DIC image depicting the cell morphology also is shown (right). (E) CLL cells were pretreated with NSC-23766 before being subjected to COLL/CXCL12. CLL cell motility (n = 5) (left); a representative DIC image (right) also is shown. (F) CLL cells after pretreatment with Y-27632 before being cultured on COLL/CXCL12. CLL cell motility (n = 5) (left) and a representative DIC image (right). All data represent means ± SD. Scale bars represent 5 µm.
CXCL12-mediated CLL cell motility is dependent on a functional Rho/ROCK signal, whereas Rac is dispensable. (A) CLL cells were cultured on immobilized CXCL12 or collagen (COLL)/CXCL12, and activation of Rac1 (left) and RhoA (right) was analyzed (n = 4). (B) Rac1 activation in CCL19-treated CLL cells (n = 4). (C) Western blot analysis of phosphorylation of ezrin/radixin/moesin proteins and Vav1 from CLL cells cultured on immobilized CXCL12 or COLL/CXCL12. A representative blot out of 3 independent experiments is shown. (D) CLL cells were subjected to COLL with or without CXCL12, and cell motility was assessed using time-lapse videomicroscopy (n = 6) (left); a representative DIC image depicting the cell morphology also is shown (right). (E) CLL cells were pretreated with NSC-23766 before being subjected to COLL/CXCL12. CLL cell motility (n = 5) (left); a representative DIC image (right) also is shown. (F) CLL cells after pretreatment with Y-27632 before being cultured on COLL/CXCL12. CLL cell motility (n = 5) (left) and a representative DIC image (right). All data represent means ± SD. Scale bars represent 5 µm.
To explore the consequences of this Rac activation defect on CLL cell polarization and motility, we next tested the phosphorylation state of key components downstream of CXCL12. Stimulation of CLL cells with a CXCL12 substrate induced the dephosphorylation of proteins of the ezrin/radixin/moesin family, which is required for correct microvillar collapse during lymphocyte polarization.29 Furthermore, CXCL12 stimulation induced the phosphorylation of the master GEF Vav1 (Figure 2C), indicating the integrity of the migratory machinery beyond Rac/Tiam signals. As conventional readouts of chemokine receptor downstream signaling, we also detected robust CXCL12-induced phosphorylation of Akt and ERK1/2 (supplemental Figure 2C).
We next sought to assess the functional contribution of the RhoA and Rac axes to CLL cell motility. Using time-lapse videomicroscopy, we addressed the ability of the Tiam1/Rac inhibitor NSC-23766 and the RhoA/ROCK antagonist Y-27632 to specifically interfere with CXCL12-induced polarization and migration of CLL cells on a collagen substrate (Figure 2D-F). We observed that the presence of collagen on its own only minimally supported random migration of CLL cells. In contrast, coimmobilization of CXCL12 robustly induced CLL cell motility on this substrate (supplemental Video 1), similar to our recent observations on hyaluronan/CCL21 substrates.17 Notably, pseudo-DIC images demonstrated the extraordinary phenotype of CLL cell polarization and motility, with its low degree of spreading (Figure 2D).
Notably, Rac activation was not involved in this migratory mode, as pretreatment with the Rac/Tiam1 inhibitor NSC-23766 did not affect polarization or motility (Figure 2E). The efficacy of this inhibitor was ensured by control experiments using NIH/3T3 cells (supplemental Figure 3A). In contrast, chemokine-induced CLL cell motility was strongly reduced in the presence of Y-27632, with complete loss of the polarized phenotype (Figure 2F), indicating the involvement of the RhoA/ROCK axis in this process. Y-27632 did not alter viability or cell size, nor did it activate CLL cells (supplemental Figure 3B). Classically, chemokine-mediated CLL cell motility was dependent on Gαi proteins and could be abrogated by pertussis toxin (supplemental Figure 3C). CLL cell motility was also diminished in the presence of PP2, a src kinase inhibitor30 (supplemental Figure 3D). It is worth mentioning that neither NSC-23766 nor Y-27632 blocked CXCL12-triggered very late antigen 4-mediated arrests under shear flow (supplemental Figure 4). These data suggest that the very first step of CLL cell extravasation, the arrest on the endothelium, likely involves GTPases distinct from both RhoA and Rac, in contrast to the RhoA usage during motility.
Collectively, our results suggest that resting CLL cells demonstrate a specific Gαi-coupled, RhoA-dependent, but Rac/Tiam1-independent mode of lateral chemokine-induced motility.
T-cell-mediated CLL cell activation results in restored Tiam1 expression
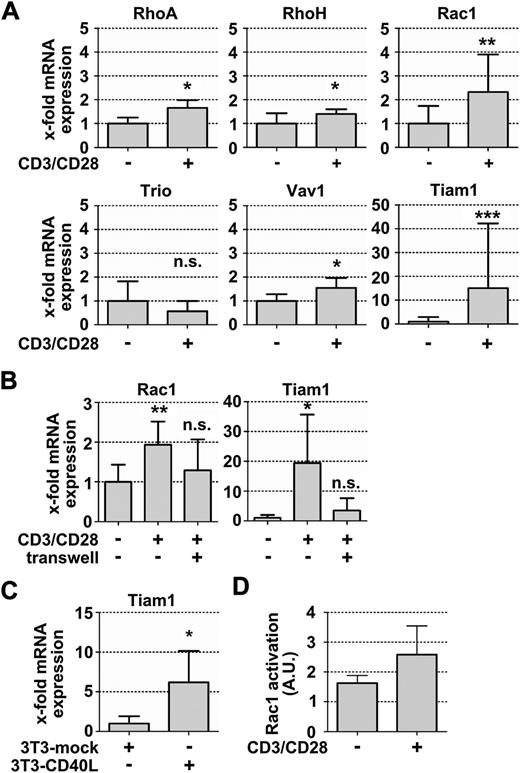
Rac is not only a master component of cellular motility but also an established element of tumor cell proliferation (for review, see Parri and Chiarugi31 ). Therefore, we next examined RhoA and Rac usage in CLL cells under conditions that mimic the situation in lymphoid CLL proliferation centers, employing our recently established in vitro system.18 There, anti-CD3/CD28 microbeads serve to activate the autologous T cells within PBMCs from patients with CLL. In turn, CLL cells receive activation signals from these activated T cells, as well as survival signals from an additional layer of fibroblasts. The activation of CLL cells, starting on 8- to 24-hour coculture, is followed by their proliferation after 3 to 5 days.18 First, to detect early activation-based alterations, we assessed the expression of the GTPases RhoA, RhoH, and Rac1 and GEFs Vav1, Trio, and Tiam1 in resting CLL cells compared with CLL cells that were activated by T cells after 24 hours. We observed slightly increased transcript levels of all investigated targets in activated CLL cells. However, only Rac and Tiam1 transcription was robustly induced on activation, with a mean upregulation of 2-fold for Rac1 and more than 15-fold for Tiam1 in activated CLL cells compared with nonactivated cells (Figure 3A), irrespective of their risk profile (supplemental Figure 5A-B). In contrast, Tiam1 transcription in normal B cells was not further increased above the nonreduced basal levels by activation (supplemental Figure 5C; supplemental Table 2; compare Figure 1E). The GEF Trio was slightly but not significantly downregulated in activated CLL cells compared with in nonactivated cells (Figure 3A). Notably, the activation-induced upregulation of Rac1 and Tiam1 in CLL cells required their direct cell–cell contact with T cells and could not be observed when activated T cells were separated from the CLL cells by a 0.4-µm pore membrane filter (Figure 3B).
Autologous T-cell activation leads to the upregulation of Rac signaling molecules in activated CLL cells. (A) Relative mRNA quantification in activated and nonactivated CLL cells using Taqman primers and probes (n = 6). (B) Relative Rac1 and Tiam1 expression after culturing CLL cells in direct cell cell-contact with autologous, anti-CD3/CD28-activated T cells or separated from the activated T cells by a 0.4-µm pore membrane filter (n = 6). (C) Relative Tiam1 mRNA expression in CLL cells that were cultured in the presence of either NIH/3T3-mock transfected fibroblasts or CD40L-transfected cells (3T3-CD40L) (n = 7). (D) Basal Rac1 activation in activated and nonactivated CLL cells, measured as described in Materials and methods (n = 3). Data represent means ± SD.
Autologous T-cell activation leads to the upregulation of Rac signaling molecules in activated CLL cells. (A) Relative mRNA quantification in activated and nonactivated CLL cells using Taqman primers and probes (n = 6). (B) Relative Rac1 and Tiam1 expression after culturing CLL cells in direct cell cell-contact with autologous, anti-CD3/CD28-activated T cells or separated from the activated T cells by a 0.4-µm pore membrane filter (n = 6). (C) Relative Tiam1 mRNA expression in CLL cells that were cultured in the presence of either NIH/3T3-mock transfected fibroblasts or CD40L-transfected cells (3T3-CD40L) (n = 7). (D) Basal Rac1 activation in activated and nonactivated CLL cells, measured as described in Materials and methods (n = 3). Data represent means ± SD.
To test whether CD40L-CD40 signals are sufficient to induce Tiam1 transcription, we next cocultured CLL cells with mock- or CD40L-transfected NIH/3T3 cells. We found upregulated Tiam1 levels in CD40-activated CLL cells comparable to the observed Tiam1 upregulation in CLL, using the T-cell activation setting (Figure 3C; supplemental Figure 5D). Next, we wished to explore the consequences of the restored Tiam1 expression on Rac1 activation. We stimulated resting and activated CLL cells with the chemokines CXCL12 or CCL19 and performed Rac1 activation assays. Activated CLL cells displayed reduced CXCR4 expression but increased CCR7 expression, rendering the CCR7 binding ligand CCL19 the relevant control chemokine for testing Rac1 activation of these cells. We found slightly elevated levels of GTP-bound Rac1 in the absence of chemokine stimulation in activated CLL cells compared with resting CLL cells (Figure 3D). However, we could not detect any further increase in GTP-bound Rac1 in activated CLL cells on their stimulation with CCL19 or CXCL12 (data not shown), suggesting a higher intrinsic rather than chemokine-inducible Rac1 activation in activated CLL cells.
Blocking Rac signaling inhibits CLL cell proliferation, which can be attributed to alterations in c-Myc transcription
To further assess the functional role of the restored Tiam1 expression and increased intrinsic Rac activation, we tested the effects of Tiam1/Rac inhibition vs RhoA/ROCK inhibition on CLL cell proliferation. We measured the number of Ki-67+ CLL cells in the presence or absence of NSC-23766 or Y-27632 and activated T cells/fibroblasts after 5 days. We found a strong, significant decrease in the number of Ki-67+ CLL cells in the presence of NSC-23766 (Figure 4A), whereas autologous T-cell proliferation was not significantly impaired (supplemental Figure 6A). ROCK inhibition by Y-27632 did not affect the number of Ki-67+ CLL cells in this setting (Figure 4B).
Autologous T-cell-mediated CLL cell proliferation is dependent on Rac. (A) Flow cytometric analysis of Ki-67-positive CLL cells after anti-CD3/CD28 bead-mediated activation with or without NSC-32766 (50 µM) pretreatment (n = 11). Data are presented in box plot format, whereas the 25th and 75th percentiles form the box, with the median marked as a line; the smallest and the largest observation form whiskers. (B) Ki-67-positive CLL cells after treatment with 10 µM Y-27632 (n = 7). (C) Relative Tiam1 gene expression in a time course experiment (left) and after 24 hours (n = 5) (right means ± SD). Dashed line, NSC-32766 treatment; solid line, control. (D) Relative c-Myc mRNA expression in a time course experiment (left) and after 24 hours (n = 5) (right means ± SD). Dashed line, NSC-32766 treatment; solid line, control. (E) Western blot analysis of Tiam1, Rac1, and c-Myc protein expression in activated and nonactivated CLL cells. Cyclophilin A (Peptidylprolyl isomerase A; PPIA) protein was used for normalization. A representative blot (left) and a densitometric analysis (right) of 3 independent experiments are shown (bars: c-Myc, light gray; Tiam1, dark gray; Rac1, black). (F) Relative mRNA expression of Tiam1 and c-Myc (left) or Rac1 and c-Myc (right) in activated CLL cells after cells were nucleofected with Tiam1-siRNA, Rac1-siRNA, or nontargeting control siRNA. One representative out of 3 independent experiments is shown.
Autologous T-cell-mediated CLL cell proliferation is dependent on Rac. (A) Flow cytometric analysis of Ki-67-positive CLL cells after anti-CD3/CD28 bead-mediated activation with or without NSC-32766 (50 µM) pretreatment (n = 11). Data are presented in box plot format, whereas the 25th and 75th percentiles form the box, with the median marked as a line; the smallest and the largest observation form whiskers. (B) Ki-67-positive CLL cells after treatment with 10 µM Y-27632 (n = 7). (C) Relative Tiam1 gene expression in a time course experiment (left) and after 24 hours (n = 5) (right means ± SD). Dashed line, NSC-32766 treatment; solid line, control. (D) Relative c-Myc mRNA expression in a time course experiment (left) and after 24 hours (n = 5) (right means ± SD). Dashed line, NSC-32766 treatment; solid line, control. (E) Western blot analysis of Tiam1, Rac1, and c-Myc protein expression in activated and nonactivated CLL cells. Cyclophilin A (Peptidylprolyl isomerase A; PPIA) protein was used for normalization. A representative blot (left) and a densitometric analysis (right) of 3 independent experiments are shown (bars: c-Myc, light gray; Tiam1, dark gray; Rac1, black). (F) Relative mRNA expression of Tiam1 and c-Myc (left) or Rac1 and c-Myc (right) in activated CLL cells after cells were nucleofected with Tiam1-siRNA, Rac1-siRNA, or nontargeting control siRNA. One representative out of 3 independent experiments is shown.
To get insight into the responsible mechanisms, we assessed the expression of c-Myc, a master regulator of proliferation. In a time course analysis, we observed that the upregulation of Tiam1 mRNA is a rapid event in T-cell-mediated CLL activation, with a maximum increase after 8 hours and a progressive decline thereafter (Figure 4C, left). As expected, inhibition of Tiam/GEF binding to Rac by NSC-23766 did not change the expression level of Tiam1 after 24 hours (Figure 4C, right). However, at the same time, Rac inhibition lead to the blockade of c-Myc induction with a maximum after 8 to 24 hours (Figure 4D, left) and significantly reduced c-Myc levels at 24 hours (Figure 4D, right). By immunoblotting, we confirmed that these transcriptional alterations were translated to the protein level (Figure 4E). CLL cell activation (CD69 expression) was not altered by the inhibition (supplemental Figure 6B), suggesting a c-Myc specific mechanism rather than a general decrease in activation responsible for the observed antiproliferative effects. As an additional control for the specificity of the used Tiam/Rac inhibitor, we used Tiam1 or Rac1 siRNA, which confirmed the necessity for Tiam1 and Rac1 in the T-cell-induced induction of c-Myc transcription that preceded CLL cell cycle induction (Figure 4F). In an additional time course experiment, the activation-mediated induction of Ki-67 mRNA was abrogated from 100-fold, compared with untreated control, to 40-fold after NSC-23766 treatment (supplemental Figure 6C).
Activated CLL cells can be resensitized toward fludarabine by Rac inhibition
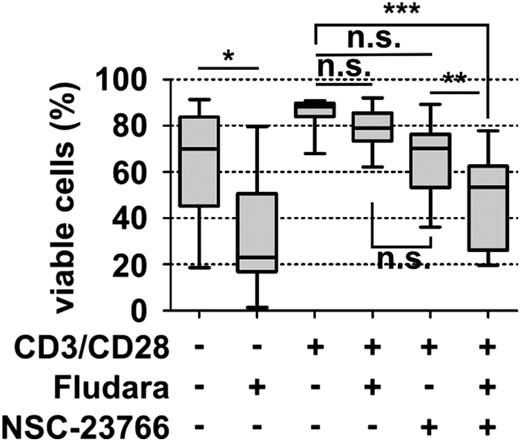
The DNA intercalating purine analog fludarabine is still the backbone of current chemotherapeutic interventions in CLL. Strikingly, fludarabine improves progression-free survival, but not overall survival,32 probably as a result of drug resistance development. For one, signals from activated T cells can contribute to fludarabine resistance of CLL cells.33 Therefore, we next investigated the contribution of Tiam1/Rac signaling to T-cell-mediated fludarabine resistance of CLL cells. After 24 hours of anti-CD3/CD28 microbead stimulation, we treated the cells with 10 µM fludarabine and/or NSC-23766 and measured cell viability after 48 hours. As expected, the cell viability of nonactivated CLL cells dropped from 63.4% to 33.7% after treatment, but activated CLL cells developed a fludarabine resistance (84.5% vs 79.0% for untreated and treated activated CLL cells, respectively). Importantly, the additional application of the Tiam1/Rac inhibitor NSC-23766 lead to the resensitization of CLL cells toward fludarabine and a cell viability rate of 49.2%. NSC-23766 alone did not induce significant cell death in activated CLL cells (Figure 5) and did not alter the viability or the extent of fludarabine-induced apoptosis of unstimulated CLL cells (supplemental Figure 7).
Rac inhibition overcomes T cell-mediated resistance toward fludarabine. CLL PBMCs were cultured with or without anti-CD3/CD28 microbeads for 24 hours before subsequent treatment with 10 µM fludarabine for an additional 48 hours. CLL cell viability was measured after 3 days, using annexin V/7-AAD staining of CD5/CD19+ cells and flow cytometric analysis (n = 12). Data are presented as box plots.
Rac inhibition overcomes T cell-mediated resistance toward fludarabine. CLL PBMCs were cultured with or without anti-CD3/CD28 microbeads for 24 hours before subsequent treatment with 10 µM fludarabine for an additional 48 hours. CLL cell viability was measured after 3 days, using annexin V/7-AAD staining of CD5/CD19+ cells and flow cytometric analysis (n = 12). Data are presented as box plots.
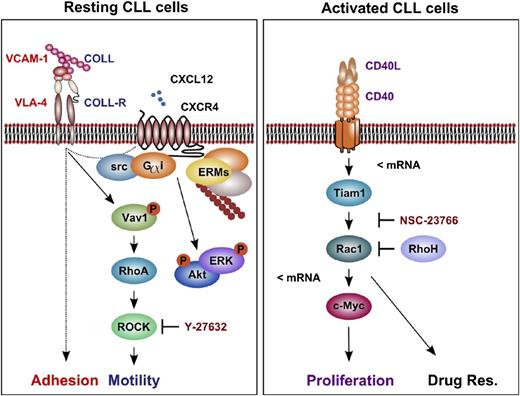
In summary, we suggest that CLL cells use a Rho/ROCK-dependent mode of cell motility but a Tiam1/Rac-dependent mode of cell proliferation and drug resistance in CLL (Figure 6).
The proposed model for RhoA/ROCK-dependent cell motility and Rac-dependent proliferation and chemoresistance in CLL. Resting, nonactivated CLL cells arrest in a RhoA/ROCK- and Rac-independent, but CXCL12-mediated, manner to VCAM-1 under shear flow. Furthermore, we observed a Gαi-, src-, and ROCK-dependent random motility of resting CLL cells on COLL/CXCL12 substrates under shear-free conditions. On their activation by T cells, we noticed a CD40-mediated transcriptional upregulation of Rac1 and Tiam1 in CLL cells, as well as Rac-dependent c-Myc transactivation and cell proliferation. Furthermore, T-cell-mediated CLL cell activation leads to a Rac-dependent resistance toward fludarabine. Dashed lines, an unknown signaling pathway; <mRNA, transcriptional regulation; COLL-R, collagen-receptor; VLA-4, integrin α4/β1 (very late antigen 4); Drug Res., drug resistance; ERM, ezrin/radixin/moesin.
The proposed model for RhoA/ROCK-dependent cell motility and Rac-dependent proliferation and chemoresistance in CLL. Resting, nonactivated CLL cells arrest in a RhoA/ROCK- and Rac-independent, but CXCL12-mediated, manner to VCAM-1 under shear flow. Furthermore, we observed a Gαi-, src-, and ROCK-dependent random motility of resting CLL cells on COLL/CXCL12 substrates under shear-free conditions. On their activation by T cells, we noticed a CD40-mediated transcriptional upregulation of Rac1 and Tiam1 in CLL cells, as well as Rac-dependent c-Myc transactivation and cell proliferation. Furthermore, T-cell-mediated CLL cell activation leads to a Rac-dependent resistance toward fludarabine. Dashed lines, an unknown signaling pathway; <mRNA, transcriptional regulation; COLL-R, collagen-receptor; VLA-4, integrin α4/β1 (very late antigen 4); Drug Res., drug resistance; ERM, ezrin/radixin/moesin.
Discussion
CLL cells cycle between the peripheral blood and lymphoid compartments. Activating signals from the lymphoid microenvironment promote the proliferation, survival, and chemoresistance of the malignant cells.34,35 In this study, we found an opposing contribution of functional RhoA and Tiam1/Rac1 to motility of resting CLL cells and microenvironment-induced survival/proliferation, respectively (Figure 5B).
To get a first insight into the role of Rho GTPases in CLL, we profiled the transcript levels of common Rho GTPases and GEFs in CLL cells compared with normal B lymphocytes. We found a severe downregulation of Tiam1 transcript levels in resting CLL cells. However, Tiam1 expression was restored once CLL cells received activating microenvironmental signals; for example, by activated autologous T lymphocytes. Tiam1 is considered an oncogene in a variety of tumors, acting as a modifier of Ras-mediated tumor transformation and progression and as a mediator of tumor cell-microenvironment interactions.36-39 A recent report suggests that a balance of Tiam1-Rac1- and RhoA-mediated signals is important for pancreatic cancer cell proliferation and invasion, respectively.40 Knocking down Tiam1 in the tumor cells increased their migratory capacity, accompanied by increased RhoA activity.40 Because Tiam1/Rac signals have previously been considered imperative for lamellipodium formation, chemokine-induced motility, and homing of multiple other nontransformed cell types,41 this observation points to a differential usage of Tiam1/Rac in cancer cells.
Correspondingly, we observed that chemokine-induced CLL cell motility was independent from Tiam1 expression and chemokine-induced Rac activation, whereas a functional RhoA/ROCK axis was mandatory. It is worth mentioning that the migratory phenotype of CLL cells on chemokine-bearing substrates was extraordinary, with a low degree of spreading compared with T lymphocytes, as previously depicted.17 This mode of migration might be the consequence of an altered cytoskeletal organization as an adaption to impaired Tiam1-induced Rac activation. This is consistent with the observation of RhoH overexpression in CLL, confirming a recent report by Troeger et al.26 Deletion of RhoH, a Rac antagonist in hematopoietic progenitor cells, lead to increased CXCL12-induced chemotaxis.42 In contrast, RhoH knockout CLL cells showed impaired CXCL12-directed migration26 that might reflect their aberrant RhoH/Rac balance. Whether other Rho GTPases, such as Rab543 or RhoG,44 compensate for impaired Tiam1/Rac1 signals and orchestrate the CLL-specific mode of migration remains to be elucidated.
An intriguing finding of our study was the contribution of restored Tiam1/Rac expression to CLL cell proliferation and chemoresistance. Mimicking the proliferation-inducing lymphoid microenvironment in 2 reductive optimized coculture setups,18 we observed the restored transcription and translation of Tiam1 protein in activated CLL cells. Little is known of the transcriptional regulation of Tiam1. Increased expression of the transcription factor AP-1 on CD40 stimulation of B cells45 might contribute to its restoration, as the Tiam1 promoter contains multiple putative AP-1 binding sites. In colon cancer subsets, Tiam1 expression can be suppressed by promoter methylation.46 At any rate, the upregulation of Tiam1, accompanied by increased Rac1 levels, preceded the induction of c-Myc and Ki-67 and the subsequent proliferation of CLL cells. c-Myc is a master regulator of cellular proliferation.47 In CLL, c-Myc expression correlates with cell cycle entry and disease progression.48-51 By using both the Tiam1/Rac inhibitor NSC-23766 as well as Tiam1 and Rac1 siRNA approaches, we demonstrated the direct dependency of c-Myc transcription on functional Tiam1 and Rac1 expression. Our suggestion of Tiam1 as a critical component of tumorigenesis is consistent with recent reports that attribute the connection of Tiam1/Rac and c-Myc to the Wnt pathway.52-54 For example, in colorectal cancer cells, a complex of Tiam1, Rac1, and the key Wnt-mediator β-catenin binds to the Wnt-responsive promoters of c-Myc and cyclin D1, thereby enhancing their transcription.53 Furthermore, Rac1 is involved in regulating the phosphorylation of the signal transducer and activator of transcription 3, which results in the transcriptional induction of c-Myc.55 It is conceivable that a similar mechanism accounts for the parallel induction of Tiam1 and c-Myc in CLL cells.
Our finding of reduced chemoresistance of CLL cells on Tiam1/Rac inhibition implies therapeutic consequences. We observed that CLL cells acquired absolute resistance toward fludarabine when cocultured with activated T cells and fibroblasts. However, they could be resensitized by Tiam1/Rac inhibition. There are several possible mechanisms by which Rac1 could contribute to an antiapoptotic outcome of activated CLL cells. First, Rac1 was shown to interact with Bcl-2 and to stabilize its antiapoptotic activity in lymphoma.56 Concurrent exposure to NSC-23766 increased the apoptotic effect of the Bcl-2 inhibitor and BH3 mimetic ABT-737 in leukemia cells.57 Bcl-2 overexpression is a hallmark of CLL58 . Coherently, BH3 mimetics have proven to be efficient in patients with CLL, but with heterogeneity,59 and in microenvironment-mediated drug resistance.60 It is possible that additional targeting of Rac could overcome some of these limitations. Second, the antiproliferative effects of Rac1 targeting have been shown in p53-deficienct lymphoma cells, which involved Akt signals.61 We recently observed that Akt targeting can overcome p53-deficiency-induced fludarabine resistance of CLL cells,62 suggesting further investigation of Rac1 and Akt signaling cascade in p53-deficient and fludarabine-resistant CLL cells.
Remarkably, described mechanisms appear to act independent of the expression of prognostic markers, such as CD38 or CD49d expression and active Tiam1/Rac1, and to be intrinsic to the general lymphoid activation of CLL cells. As a consequence, the use of Rac targeting approaches could be a risk group-independent therapeutic strategy to selectively target the proliferative and resistant pool of CLL cells, which is considered responsible for recurrent relapses of the patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all patients for participation, Karin Stefanon for sample preparation, and Dr Andrea Zurl for providing access to the multiplate reader. This work has been supported by the Austrian Science Fund FWF (SFB-P021 to R.G. and P25015-B13 to T.N.H.), the Paracelsus Medical University Salzburg (PMU-FFF E-12/15/074-HAH and E-10/11/058-HAR to T.N.H.), the Salzburg Cancer Research Institute - Laboratory for Immunological and Molecular Cancer Research GmbH, and the province of Salzburg.
Authorship
Contribution: T.N.H., S.W.H., and R.G. designed research; S.W.H., P.W.K., S.G., K.O., U.P., and M.W., performed research; S.W.H., T.N.H., P.W.K., D.A., H.K., R.H., and R.G. analyzed and interpreted data; and S.W.H. and T.N.H. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tanja N. Hartmann, Laboratory for Immunological and Molecular Cancer Research, Third Medical Department with Hematology, Oncology, Hemostaseology, Infectiology, and Rheumatology, Paracelsus Medical University, Salzburg, Austria; e-mail: t.hartmann@salk.at.