Key Points
von Willebrand factor in its glycoprotein Ib conformation predicts 28-day mortality and could be a useful addition to the APACHE-IV score.
Abstract
Endothelial dysfunction contributes to the pathology of systemic inflammatory response syndrome (SIRS). However, endothelial biomarkers are not routinely evaluated in this setting. Here, 275 patients with SIRS and plasma levels of von Willebrand factor (VWF), thrombospondin-1, myeloperoxidase, ADAMTS-13, and active VWF (aVWF) were studied in relation to 28-day mortality. On admission, aVWF levels were higher in nonsurvivors vs survivors (0.69 vs 0.47 µg/mL, P = .019). Patients in the highest tertile of aVWF levels had a lower cumulative survival (86% vs 75%, P = .017) and twofold increased hazard ratio (HR). When adjusted for the Acute Physiology and Chronic Health Evaluation IV (APACHE-IV) score, this difference remained significant (HR 1.82, 95% confidence interval, 1.03-3.3). On admission, no significant differences were measured for the other proteins. These observations suggest that the stimulated release of VWF is not predictive for mortality in patients with SIRS, opposite of the processing of VWF after release. aVWF could be used with the APACHE-IV score to stratify SIRS patients at high mortality risk.
Introduction
Systemic inflammatory response syndrome (SIRS) occurs frequently in patients who present to intensive care units (ICUs).1 Because endothelial activation is a hallmark of SIRS and sepsis, endothelial markers could provide important prognostic information.2 A powerful thrombogenic molecule released from Weibel-Palade bodies of endothelial cells (ECs) upon their activation is von Willebrand factor (VWF).3 VWF captures and localizes platelets to the vessel wall.4 In SIRS and sepsis, this frequently leads to massive aggregation of platelets at the endothelial surface and subsequently to thrombocytopenia and consumptive coagulopathy.5 Regarded as a gold marker of endothelial activation,6 VWF levels were reported to be 11 times higher in patients with SIRS compared with controls and were postulated to reflect generalized endothelial activation.7 High VWF levels were also found in patients with severe sepsis or septic shock when compared with healthy controls, where it was suggested that VWF might predict survival.8 Nevertheless, the prognostic value of VWF in SIRS and sepsis patients remains controversial.9 A possible explanation could be that VWF in plasma is inactive, in contrast to the active conformation upon release from ECs.10 Here, in addition to VWF, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS-13), myeloperoxidase (MPO), and thrombospondin (TSP), active VWF (aVWF) was measured and its prognostic value was evaluated in a cohort of patients with SIRS.
Materials and methods
The study design was described previously.11 In brief, 275 patients with an anticipated length of stay of more than 24 hours whose symptoms fulfilled at least 2 SIRS criteria at admission to the ICU were included.11 Blood samples drawn during the first 10 days in the ICU were available for measurements. Levels of VWF, TSP, and ADAMTS-13 were measured as described before.12,13 MPO and aVWF were measured using a semiautomated enzyme-linked immunosorbent assay (ELISA) on a TECAN Freedom EVO robot (Tecan, Männedorf, Switzerland). The homemade antibody against aVWF (5 μg/mL) or antibodies against MPO (2 μg/mL; R&D Systems, Minneapolis, MN) were coated overnight at 4°C in 384-well Nunc maxisorp plates (Nunc, Roskilde, Denmark). The antibodies were then washed in phosphate-buffered saline (PBS)/0.5% Tween. After blocking with 2% bovine serum antigen/PBS for 2 hours, plasma samples were diluted in PBS/1% bovine serum antigen (10× for aVWF and 4× for MPO) and incubated for an additional 2 hours. In the aVWF assay, a calibration curve was obtained by diluting recombinant VWF type 2B (R1306Q) in VWF-deficient plasma, which was produced as described previously.14 The level of aVWF in plasma pooled from 40 healthy individuals was measured at 0.1 μg/mL. Recombinant MPO was used to make a calibration curve for MPO. Unbound fractions were washed again 5 times in PBS/0.5%Tween. For aVWF detection, a polyclonal horseradish peroxidase (HRP)-labeled antibody against VWF (Dako, Glostrup, Denmark) was used and washing in PBS as described earlier followed. For MPO, a biotinylated goat anti-human MPO (0.2 μg/mL; R&D Systems) and streptavidine-HRP (Dako) were used for detection. Relative light units from HRP activity were measured with a chemiluminescent substrate (super-signal ELISA pico chemiluminescent substrate; Thermo Fisher Scientific, Waltham, MA) by Spectramax reader (MDS Analytical Technologies/Molecular Devices, Sunnyvale, CA). Statistical analyses were performed with SPSS 20.0 (SPSS Inc., Armonk, NY). Data are presented as medians or counts and proportions (%). Continuous data were compared with the Mann-Whitney U test. Patients were categorized as having high aVWF levels if they had values in the third tertile. The Acute Physiology and Chronic Health Evaluation IV (APACHE-IV) score was dichotomized at the median value, which was 75. The cumulative survival was calculated by applying the Kaplan-Meier method, and differences in mortality were compared with the log-rank test. Univariate and multivariate Cox regression proportional hazards analyses were done to study the effects on outcome. A 2-tailed P < .05 was considered to indicate statistical significance. The study was approved by the institutional review board of the University Medical Center Utrecht and it waived the need for informed consent (University Medical Center Utrecht Institutional Review Board research protocol 108-188). The study was conducted in accordance with the Declaration of Helsinki.
Results and discussion
Demographic and clinical characteristics of survivors and nonsurvivors are shown in Table 1. aVWF was higher in nonsurvivors vs survivors on days 1 through 3. VWF levels were increased but not different between the groups. At admission, levels of ADAMTS-13, MPO, and TSP were also not different between the groups (Figure 1 and Table 2). An inverse correlation between ADAMTS-13 levels and VWF levels was observed during the 10-day period of measurements. ADAMTS-13 correlated inversely, albeit weakly, with aVWF only at day 3 (R = −0.13, P = .04).
Demographic and clinical baseline characteristics in survivors and nonsurvivors
| Characteristic . | Survivors (n = 228) . | Nonsurvivors (n = 47) . |
|---|---|---|
| Age, y (IQR) | 61 (50-74) | 72 (60-76)* |
| Gender (male) | 144 (63%) | 29 (61%) |
| Referring specialty | ||
| Cardiology | 13 (5%) | 11 (23%) |
| Internal medicine | 39 (17%) | 14 (29%) |
| Neurology | 23 (10%) | 3 (6%) |
| Neurosurgery | 22 (10%) | 5 (10%) |
| Cardiopulmonary surgery | 19 (8%) | 3 (6%) |
| Surgery | 78 (34%) | 7 (15%) |
| Other | 34 (15%) | 4 (8%) |
| Inflammation/infection | ||
| Proven infection | 89 (39%) | 22 (46%)* |
| Sepsis | 5 (2%) | 0 (0%) |
| Severe sepsis | 44 (19%) | 7 (15%) |
| Septic shock | 40 (18%) | 15 (32%)† |
| Body temperature, °C (SD) | 38.2 (±1.1) | 38.1 (±1.5) |
| CRP, mg/mL (IQR) | 185 (106-281) | 184 (101-306) |
| PCT, μg/mL (IQR) | 1.15 (0.35-4.42) | 4.04 (0.59-9.8)* |
| Severity of illness | ||
| Mechanical ventilation | 146 (64%) | 28 (60%) |
| APACHE-IV score (IQR) | 72 (56-92) | 117 (96-136)† |
| Hemostasis | ||
| Prothrombin time, s (IQR) | 17.3 (15.5-22.6) | 14.8 (14.4-18) |
| Platelet count, × 109/L (IQR) | 178 (115-262) | 170 (90-336) |
| D-dimer, μg/mL (IQR) | 3.0 (1.8-5.1) | 4.6 (2.9-5.6) |
| Characteristic . | Survivors (n = 228) . | Nonsurvivors (n = 47) . |
|---|---|---|
| Age, y (IQR) | 61 (50-74) | 72 (60-76)* |
| Gender (male) | 144 (63%) | 29 (61%) |
| Referring specialty | ||
| Cardiology | 13 (5%) | 11 (23%) |
| Internal medicine | 39 (17%) | 14 (29%) |
| Neurology | 23 (10%) | 3 (6%) |
| Neurosurgery | 22 (10%) | 5 (10%) |
| Cardiopulmonary surgery | 19 (8%) | 3 (6%) |
| Surgery | 78 (34%) | 7 (15%) |
| Other | 34 (15%) | 4 (8%) |
| Inflammation/infection | ||
| Proven infection | 89 (39%) | 22 (46%)* |
| Sepsis | 5 (2%) | 0 (0%) |
| Severe sepsis | 44 (19%) | 7 (15%) |
| Septic shock | 40 (18%) | 15 (32%)† |
| Body temperature, °C (SD) | 38.2 (±1.1) | 38.1 (±1.5) |
| CRP, mg/mL (IQR) | 185 (106-281) | 184 (101-306) |
| PCT, μg/mL (IQR) | 1.15 (0.35-4.42) | 4.04 (0.59-9.8)* |
| Severity of illness | ||
| Mechanical ventilation | 146 (64%) | 28 (60%) |
| APACHE-IV score (IQR) | 72 (56-92) | 117 (96-136)† |
| Hemostasis | ||
| Prothrombin time, s (IQR) | 17.3 (15.5-22.6) | 14.8 (14.4-18) |
| Platelet count, × 109/L (IQR) | 178 (115-262) | 170 (90-336) |
| D-dimer, μg/mL (IQR) | 3.0 (1.8-5.1) | 4.6 (2.9-5.6) |
Data are presented as counts and percentages unless stated otherwise. Body temperature is presented as mean with standard deviation.
APACHE-IV, Acute Physiology and Chronic Health Evaluation IV severity of illness model; CRP, C-reactive protein; SD, standard deviation; s, seconds; y, year.
P < .05.
P < .01.
Plasma levels. (A) VWF, (B) aVWF, (C) ADAMTS-13, (D) MPO, and (E) TSP levels in survivors and nonsurvivors during their first 10 days in the ICU. Data are presented as medians (*P < .05).
Plasma levels. (A) VWF, (B) aVWF, (C) ADAMTS-13, (D) MPO, and (E) TSP levels in survivors and nonsurvivors during their first 10 days in the ICU. Data are presented as medians (*P < .05).
VWF, aVWF, ADAMTS-13, MPO, and TSP values during the first 10 days in the ICU
| . | VWF, μg/mL (IQR) . | aVWF, μg/mL (IQR) . | ADAMTS-13, μg/mL (IQR) . | MPO, ng/mL (IQR) . | TSP, ng/mL (IQR) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Survivors . | Nonsurvivors . | Survivors . | Nonsurvivors . | Survivors . | Nonsurvivors . | Survivors . | Nonsurvivors . | Survivors . | Nonsurvivors . |
| Day 1, N = 228; 47 | 46.0 (31.3-63.9) | 50.1 (29.6-80.8) | 0.47 (0.30-0.82) | 0.69 (0.35-1.68) | 0.46 (0.31-0.68) | 0.40 (0.22-0.62) | 46 (23-75) | 52 (32-93) | 515 (809-294) | 428 (266-718) |
| Day 2, N = 224; 46 | 46.7 (32.2-68.3) | 57.6 (38.0-87.8) | 0.51 (0.29-0.83) | 0.79 (0.41-1.44) | 0.49 (0.31-0.71) | 0.34 (0.20-0.62) | 37 (21-64) | 49 (21-107) | 495 (220-753) | 388 (257-699) |
| Day 3, N = 208; 38 | 51.5 (34.7-72.3) | 50.9 (37.3-109.3) | 0.50 (0.34-0.86) | 1.02 (0.55-1.25) | 0.48 (0.29-0.76) | 0.31 (0.14-0.48) | 36 (20-61) | 52 (25-100) | 573 (281-895) | 469 (199-646) |
| Day 4, N = 187; 34 | 49.9 (34.0-68.1) | 51.1 (34.7-83.7) | 0.51 (0.31-0.94) | 0.71 (0.47-1.35) | 0.44 (0.28-0.69) | 0.31 (0.18-0.53) | 33 (17-52) | 46 (24-66) | 538 (287-763) | 418 (174-718) |
| Day 5, N = 170; 31 | 50.6 (38.4-67.8) | 50.5 (38.7-81.9) | 0.56 (0.36-0.97) | 0.76 (0.36-1.19) | 0.43 (0.24-0.72) | 0.28 (0.19-0.26) | 29 (18-51) | 28 (17-62) | 545 (251-841) | 372 (190-780) |
| Day 6, N = 155; 28 | 51.1 (36.3-67.8) | 60.6 (41.3-102.4) | 0.59 (0.36-1.03) | 0.62 (0.41-1.02) | 0.38 (0.25-0.68) | 0.29 (0.17-0.49) | 27 (14-46) | 35 (19-66) | 522 (258-799) | 381 (196-626) |
| Day 7, N = 141; 23 | 51.7 (37.5-74.0) | 56.2 (35.5-121.2) | 0.55 (0.35-1.23) | 0.58 (0.44-1.09) | 0.36 (0.24-0.64) | 0.28 (0.19-0.50) | 26 (17-43) | 43 (23-61) | 531 (289-867) | 342 (157-517) |
| Day 8, N = 127; 16 | 51.8 (37.1-73.3) | 58.3 (38.4-120.1) | 0.65 (0.33-1.00) | 0.62 (0.43-1.21) | 0.38 (0.24-0.59) | 0.29 (0.15-0.65) | 24 (14-44) | 41 (30-72) | 463 (234-717) | 396 (244-658) |
| Day 9, N = 107; 12 | 53.0 (36.5-79.0) | 58.8 (47.0-88.7) | 0.56 (0.38-1.16) | 0.58 (0.45-0.85) | 0.40 (0.19-0.69) | 0.22 (0.14-0.38) | 24 (14-48) | 60 (40-75) | 441 (259-697) | 598 (361-783) |
| Day, 10 N = 85; 5 | 51.1 (36.7-72.7) | 45.0 (43.5-93.4) | 0.56 (0.40-0.95) | 0.59 (0.48-0.85) | 0.39 (0.24-0.70) | 0.29 (0.11-0.35) | 20 (14-46) | 66 34-68) | 478 (267-678) | 460 (281-771) |
| . | VWF, μg/mL (IQR) . | aVWF, μg/mL (IQR) . | ADAMTS-13, μg/mL (IQR) . | MPO, ng/mL (IQR) . | TSP, ng/mL (IQR) . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Survivors . | Nonsurvivors . | Survivors . | Nonsurvivors . | Survivors . | Nonsurvivors . | Survivors . | Nonsurvivors . | Survivors . | Nonsurvivors . |
| Day 1, N = 228; 47 | 46.0 (31.3-63.9) | 50.1 (29.6-80.8) | 0.47 (0.30-0.82) | 0.69 (0.35-1.68) | 0.46 (0.31-0.68) | 0.40 (0.22-0.62) | 46 (23-75) | 52 (32-93) | 515 (809-294) | 428 (266-718) |
| Day 2, N = 224; 46 | 46.7 (32.2-68.3) | 57.6 (38.0-87.8) | 0.51 (0.29-0.83) | 0.79 (0.41-1.44) | 0.49 (0.31-0.71) | 0.34 (0.20-0.62) | 37 (21-64) | 49 (21-107) | 495 (220-753) | 388 (257-699) |
| Day 3, N = 208; 38 | 51.5 (34.7-72.3) | 50.9 (37.3-109.3) | 0.50 (0.34-0.86) | 1.02 (0.55-1.25) | 0.48 (0.29-0.76) | 0.31 (0.14-0.48) | 36 (20-61) | 52 (25-100) | 573 (281-895) | 469 (199-646) |
| Day 4, N = 187; 34 | 49.9 (34.0-68.1) | 51.1 (34.7-83.7) | 0.51 (0.31-0.94) | 0.71 (0.47-1.35) | 0.44 (0.28-0.69) | 0.31 (0.18-0.53) | 33 (17-52) | 46 (24-66) | 538 (287-763) | 418 (174-718) |
| Day 5, N = 170; 31 | 50.6 (38.4-67.8) | 50.5 (38.7-81.9) | 0.56 (0.36-0.97) | 0.76 (0.36-1.19) | 0.43 (0.24-0.72) | 0.28 (0.19-0.26) | 29 (18-51) | 28 (17-62) | 545 (251-841) | 372 (190-780) |
| Day 6, N = 155; 28 | 51.1 (36.3-67.8) | 60.6 (41.3-102.4) | 0.59 (0.36-1.03) | 0.62 (0.41-1.02) | 0.38 (0.25-0.68) | 0.29 (0.17-0.49) | 27 (14-46) | 35 (19-66) | 522 (258-799) | 381 (196-626) |
| Day 7, N = 141; 23 | 51.7 (37.5-74.0) | 56.2 (35.5-121.2) | 0.55 (0.35-1.23) | 0.58 (0.44-1.09) | 0.36 (0.24-0.64) | 0.28 (0.19-0.50) | 26 (17-43) | 43 (23-61) | 531 (289-867) | 342 (157-517) |
| Day 8, N = 127; 16 | 51.8 (37.1-73.3) | 58.3 (38.4-120.1) | 0.65 (0.33-1.00) | 0.62 (0.43-1.21) | 0.38 (0.24-0.59) | 0.29 (0.15-0.65) | 24 (14-44) | 41 (30-72) | 463 (234-717) | 396 (244-658) |
| Day 9, N = 107; 12 | 53.0 (36.5-79.0) | 58.8 (47.0-88.7) | 0.56 (0.38-1.16) | 0.58 (0.45-0.85) | 0.40 (0.19-0.69) | 0.22 (0.14-0.38) | 24 (14-48) | 60 (40-75) | 441 (259-697) | 598 (361-783) |
| Day, 10 N = 85; 5 | 51.1 (36.7-72.7) | 45.0 (43.5-93.4) | 0.56 (0.40-0.95) | 0.59 (0.48-0.85) | 0.39 (0.24-0.70) | 0.29 (0.11-0.35) | 20 (14-46) | 66 34-68) | 478 (267-678) | 460 (281-771) |
Data are presented as medians. IQR is between the 25th and 75th percentiles.
N, numbers of survivors and nonsurvivors.
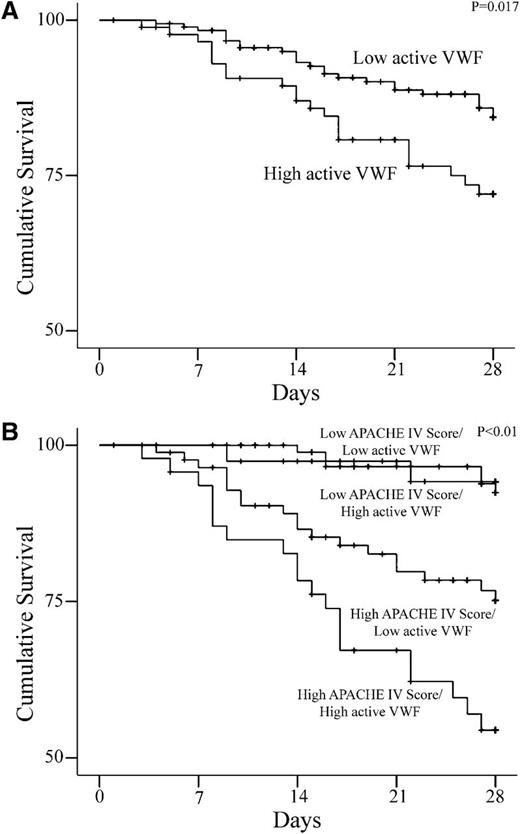
Patients with high aVWF levels on admission had lower cumulative survival vs patients with low aVWF levels (75 vs 86%, P = .017; Figure 2A) and a twofold increased hazard ratio (HR, 1.98; 95% confidence interval [CI], 1.1-3.5]. When adjusted for the APACHE-IV score, this association remained significant (HR, 1.82; 95% CI, 1.03-3.3). Compared with patients with a low APACHE-IV score, patients with both a high APACHE-IV score and high aVWF levels had a HR of 9 (95% CI, 4.0-20.5). Patients with high aVWF levels and a high APACHE-IV score also had lower cumulative survival when compared with the balance (Figure 2B). A weak correlation between aVWF and the APACHE-IV score was observed (R = 0.14, P = .02). On admission, differences in mortality rate in patients with ADAMTS-13 levels in the highest tertile compared with those with lower ADAMTS-13 levels were not observed. On day 3, however, patients with ADAMTS-13 levels in the highest tertile showed a fourfold decreased mortality rate compared with patients with ADAMTS-13 levels in the lowest tertile (HR, 0.22; 95% CI, 0.07-0.74). Patients with severe septic shock or severe sepsis did not have higher aVWF levels when compared with patients with SIRS. No correlation between aVWF levels and platelet counts was observed on admission.
Kaplan-Meier estimates of cumulative survival (in percentages) for patients. (A) aVWF plasma levels in the highest tertile compared with the lower 2 tertiles. (B) Patients with both high aVWF and high APACHE score compared with other patients.
Kaplan-Meier estimates of cumulative survival (in percentages) for patients. (A) aVWF plasma levels in the highest tertile compared with the lower 2 tertiles. (B) Patients with both high aVWF and high APACHE score compared with other patients.
Total VWF levels were higher in patients who had a proven infection during the first 10 days in the hospital (40.3 μg/mL; interquartile range [IQR] 29.7-57.8 for patients with no infection vs 50.8 μg/mL, IQR 35.4-84.0 for patients with infection; P < .01.) The inverse was observed for ADAMTS-13 levels, where higher levels were observed in patients without a proven infection (0.53 μg/mL, IQR 0.33-0.77 vs 0.34 μg/mL, IQR 0.21-0.62; P < .01).
This study shows that SIRS patients with high aVWF levels on admission have a twofold higher mortality rate independent from the APACHE-IV score. As observed previously,7 total VWF levels were increased fivefold in the entire patient cohort. However, in contrast to aVWF, total VWF failed to predict mortality. These observations are in agreement with previously published findings.15
It is suggested that VWF is released from ECs in an active conformation10 but thereafter is rapidly cleared by low density lipoprotein receptor-related protein 1.16,17 Although ADAMTS-13 was not shown to be involved in the process of clearance,18 there is evidence that proteolysis of multimeric VWF by ADAMTS-13 also leads to the inaccessibility of the A1 domain.10 In this study, a strong relation between aVWF and ADAMTS-13 was not observed. Granulocyte-derived proteases such as granzymes B and M are also involved in the “in-activation” of aVWF19 and could partly replace ADAMTS-13 in modifying aVWF at some point during the course of the disease. To elucidate this, MPO, a granulocyte activation marker,20 was measured. Similar to ADAMTS-13, MPO levels did not correlate with aVWF levels. A possible explanation for this could be that MPO also mediates oxidation of VWF through the production of hypochlorous acid and therefore inhibits its cleavage by ADAMTS-13.21 A third factor that has been shown to be involved in the processing of VWF is TSP.22 However, a significant decrease of TSP in nonsurvivors was observed on day 3 only. Taken together, these results support the suggestion that clearance,17 rather than regulation of activity, is the primary mechanism responsible for controlling aVWF levels.
In summary, this study shows that in SIRS and sepsis, aVWF is associated with mortality independently from the APACHE-IV score. Although aVWF could be a useful addition to the APACHE-IV score, further studies are warranted to determine whether there is a causal relationship between aVWF and mortality in patients with SIRS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Matthijs Linssen and Brechtje van der Velden for their technical assistance.
This work was supported by the Center for Translational Molecular Medicine (project INCOAG 01C-201) and the Dutch Heart Foundation (A.H.).
Authorship
Contribution: A.H., D.W.d.L., P.G.d.G., J.K., and M.R. designed the study; A.H. performed research; A.H., H.K., and D.W.d.L. collected data; A.H., D.W.L., P.G.d.G., and M.R. analyzed and interpreted the data; A.H., D.W.d.L., and M.R. performed statistical analysis; A.H. drafted the article; H.K., D.W.d.L., J.K., P.G.d.G., and M.R. revised the article critically for important intellectual content; and A.H., H.K., D.W.d.L., J.K., P.G.d.G., and M.R. gave final approval of the manuscript to be published.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philip G. de Groot, University Medical Center Utrecht, Department of Clinical Chemistry and Haematology (G03.550), Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; e-mail: ph.g.degroot@umcutrecht.nl.