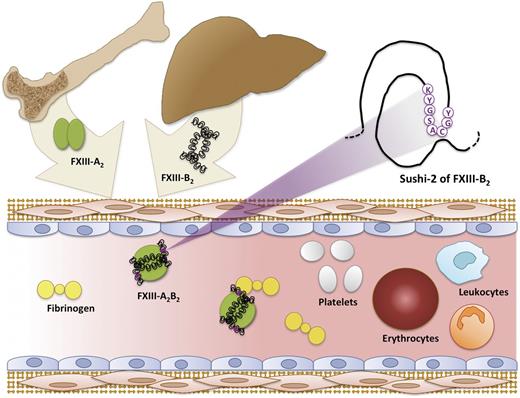
FXIII-A2 and FXIII-B2 syntheses occur in cells of bone marrow origin and liver, respectively. The FXIII-A2B2 complex assembles in the plasma, where it subsequently circulates in a complex with fibrinogen.
FXIII-A2 and FXIII-B2 syntheses occur in cells of bone marrow origin and liver, respectively. The FXIII-A2B2 complex assembles in the plasma, where it subsequently circulates in a complex with fibrinogen.
Interest in the transglutaminase FXIII is experiencing a resurgence. Best known for its effects on clot stability, FXIII has also demonstrated diverse functions in wound healing, pregnancy, angiogenesis, and inflammation/infection. It is surprising, then, how much of the biochemistry governing FXIII structure and biological distribution remains unknown.
FXIII is one of 9 members of a family of proteins found in both cellular (transglutaminases 1-7 and erythrocyte band 4.2) and plasma (pFXIII) compartments. Eight of these proteins produce inter- and intramolecular ε-N-(γ-glutamyl)-lysine bonds to crosslink proteins and protein complexes; a ninth protein, erythrocyte band 4.2, has similar domain organization but lacks a catalytic cysteine residue essential for the transglutaminase active site.2 Although transglutaminase family members have only modest sequence similarity, they share a common domain organization consisting of a β-sandwich domain, a catalytic domain, and 2 β-barrel domains (Mr 83 kDa).
FXIII is unique because it circulates in plasma as 2 catalytic subunits (FXIII-A2) and 2 noncatalytic subunits (FXIII-B2) arranged in a noncovalent heterotetramer (FXIII-A2B2, Mr 325 kDa). The noncatalytic B subunit (Mr 80 kDa) is a glycoprotein consisting of 10 sushi domains. Formation of the FXIII-A2B2 complex is critical because, in the absence of the B subunit, the A subunit is slowly spontaneously activated. The B subunit protects the A subunit from proteolysis and therefore prolongs its circulating half-life.3 Patients with B subunit deficiency experience mild bleeding in spite of apparently normal synthesis of the catalytic A subunit. Interestingly, because the A subunit is synthesized in cells of bone marrow origin and the B subunit is synthesized in the liver, formation of the FXIII-A2B2 complex must occur in plasma (see figure). The ability of FXIII-A2 to bind FXIII-B2 in plasma provides a biochemical rationale for the use of intravenous recombinant FXIII-A2 in A subunit–deficient patients; infused recombinant FXIII-A2 rapidly binds circulating FXIII-B2, creating a stable circulating complex. Clearly, delineating the mechanism regulating the interaction between these subunits is important on both basic biochemical and clinical levels.
The present study by Katona et al starts not with their first step forward but with a thoughtful step back. Two groups previously used enzyme-linked immunosorbent assay–type binding assays to investigate the affinity of the A and B subunit interaction and calculated affinity constants within an order of magnitude of each other between 4 × 10−7 M and 8 × 10−8 M.4,5 This degree of consistency is typically regarded as an acceptable level of scientific validation, and these values have stood for 10 or more years. However, Katona et al noted discord between these values and empiric measurements of the FXIII-A2 subunit concentration in plasma. Given the plasma concentrations of the A and B subunits, these affinity constants imply that most FXIII-A2 should circulate in the free form, whereas in reality, almost all plasma FXIII-A2 is present in complex with FXIII-B2. Thus, these values must substantially underestimate the affinity of the A and B subunits. The revised affinity constant determined in the present work (∼10−10 M) is significantly tighter than previous values and is supported by independent and creative tests provided by the natural biodistribution of FXIII. Using the newly determined affinity constant, Katona et al predict and then confirm experimentally that 99% of plasma FXIII-A2 is present in complex with FXIII-B2 and only ∼1% of circulating FXIII-A2 is present as free homodimers. In contrast, in 2 nonplasma sources of FXIII-A2 (cerebrospinal fluid and tears) in which FXIII-subunit concentrations are 3 orders of magnitude lower than in plasma, >80% of FXIII-A2 is present in the free form. Use of these 2 biological pools of FXIII not only validates the newly calculated affinity measurement but also shows that FXIII is uniquely regulated in different fluids.
The present study also sheds light on a second unresolved question regarding the identity of FXIII-B subunit residues that mediate its association with the FXIII-A subunit. In a 2-step sequence of experiments, Katona et al first demonstrate that a monoclonal antibody recognizing sushi domains 1 and 2 of the FXIII-B subunit prevents complex formation with the FXIII-A subunit, consistent with previous data implicating the first FXIII-B sushi domain in FXIII-A subunit binding.6 Surprisingly, however, their subsequent experiments localize the epitope to amino acid residues 90 to 103 on the second sushi domain (see figure). As the authors note, these findings are not necessarily contradictory to the earlier study and may reflect complex interactions between the first and second sushi domains of the FXIII-B subunit. Additional studies using peptides and constructs expressing amino acid mutations within this region are needed to resolve the relationship between these residues and FXIII-A2B2 complex formation.
In addition to providing fundamental information about mechanisms regulating FXIII-A2B2 heterotetramer assembly, the observation that FXIII concentration and quaternary structure differ in intra- and extravascular fluids raises new questions about its regulation and function in these spaces. Why is FXIII-B2 present in excess in these fluids? Does free FXIII-B2 have a function(s) apart from its role in the FXIII-A2B2 complex? Is FXIII-A2 in tears and cerebrospinal fluid prone to spontaneous activation, and is basal constitutive FXIII-A2 activation beneficial in tears and cerebrospinal fluid? The thoughtful approach taken by Katona et al for the determination and confirmation of their measurements is a model for the type of well-designed and executed biological investigations needed to resolve these outstanding questions.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal