Key Points
Safe mobilization of CD34+ cells in adults with β-thalassemia and effective transduction with a globin vector under cGMP conditions.
Stable vector copy number and β-globin expression in BFU-Es derived from engrafted CD34+ HPCs 6 months post-transplant in NSG mice.
Abstract
We conducted a pilot trial to investigate the safety and effectiveness of mobilizing CD34+ hematopoietic progenitor cells (HPCs) in adults with β-thalassemia major. We further assessed whether thalassemia patient CD34+ HPCs could be transduced with a globin lentiviral vector under clinical conditions at levels sufficient for therapeutic implementation. All patients tolerated granulocyte colony-stimulating factor well with minimal side effects. All cell collections exceeded 8 × 106 CD34+ cells/kg. Using clinical grade TNS9.3.55 vector, we demonstrated globin gene transfer averaging 0.53 in 3 validation runs performed under current good manufacturing practice conditions. Normalized to vector copy, the vector-encoded β-chain was expressed at a level approximating normal hemizygous protein output. Importantly, stable vector copy number (0.2-0.6) and undiminished vector expression were obtained in NSG mice 6 months posttransplant. Thus, we validated a safe and effective procedure for β-globin gene transfer in thalassemia patient CD34+ HPCs, which we will implement in the first US trial in patients with severe inherited globin disorders. This trial is registered at www.clinicaltrials.gov as #NCT01639690.
Introduction
The β-thalassemias are hereditary anemias caused by the deficient production of the β-chain of hemoglobin.1 The standard of care for patients with β-thalassemia major consists in lifelong transfusion therapy combined with pharmacologic iron chelation.1-3 The only curative treatment is allogeneic bone marrow transplantation from a matched, related donor.4,5 Most patients, however, lack such matched donor.6 The goal of therapeutic globin gene transfer is to stably insert a functional globin gene into the patient’s own hematopoietic progenitor cells (HPCs) to achieve transfusion independence.7 We previously demonstrated successful globin gene therapy in murine thalassemia models, using a lentiviral vector that includes the human β-globin promoter and arrayed regulatory elements uniquely combined to achieve high level and erythroid-specific globin expression.8-10 The vector termed TNS9 increased hemoglobin levels by an average 4 to 6 g/dL per vector copy.8-10 Several groups have confirmed and extended these results in models of thalassemia and sickle cell disease, using variant vectors encoding β-, γ-, or mutated β-globin genes.7,11,12 For the past decade, the inability to transduce patient CD34+ HPCs at potentially therapeutic levels under clinically relevant conditions has precluded effective implementation of this therapy.12-15
Study design
CD34+ cell collection and clinical grade TNS9.3.55 vector stocks
We used granulocyte colony-stimulating factor (G-CSF) (10 µg/kg, once daily subcutaneously for 6 days) to mobilize HPCs as specified in the Memorial Sloan-Kettering Cancer Center’s Institutional Review Board-approved protocol. This study was conducted in accordance with the Declaration of Helsinki. CD34+ cells were selected using an ISOLEX TM 300i (patients 1-3) or CliniMacs system (patients 4-5). Clinical grade and GLP TNS9.3.55 vector stocks, manufactured under current good manufacturing practice (cGMP) conditions at the Center for Biomedicine and Genetics (CBG, Duarte, CA) had a HeLa titer of 3.5 and 6.6 × 108 TU/mL, respectively.
Transduction and VCN quantification
CD34+ HPCs were cultured for 18 to 24 hours in serum-free X-VIVO 10 supplemented with human stem cell factor, Fms-like tyrosine kinase 3 ligand (Flt3-L), thrombopoietin, and interleukin-3. Fractions were subsequently cultured for 14 to 16 days in liquid erythroid cultures (see supplemental Methods available on the Blood Web site) or hematopoietic colony assays for vector copy number (VCN) quantification by quantitative polymerase chain reaction using the Applied Biosystems 7500 real-time polymerase chain reaction system (see supplemental Methods for details).
Analysis of human cells engrafted in NSG mice
Murine studies were conducted under a Memorial Sloan-Kettering Cancer Center’s Institutional Animal Care and Use Committee-approved protocol. Non-obese diabetic (NOD) Cg-Prkdcscid IL2R-null (NOD/severe combined immunodeficiency-γ-null, NSG) mice were conditioned with 35 mg/kg busulfan 24 hours prior to receiving TNS9.3.55-transduced HPCs. Bone marrow was analyzed 3.5 to 7 months posttransplantation (see supplemental Methods for details).
Globin expression studies
Globin chain expression was analyzed by high-performance liquid chromatography as previously described. Total RNA was isolated from peripheral blood and from erythroid burst-forming units (BFU-Es) generated from pre-infusion CD34+ cell cultures or posttransplant NSG bone marrow. Primers and probes were previously described (see supplemental Methods for details).
Results and discussion
Here, we demonstrate safe and efficacious CD34+ cell collection in transfusion-dependent β-thalassemia major patients and robust globin gene transfer under cGMP conditions. All 5 enrolled adults were on a hypertransfusion and chelation regimen (supplemental Table 1). Throughout the 6-day mobilization process, the maximum white blood cell counts and absolute neutrophil counts reached 46 to 65 × 109/L and 43 to 55 × 109/L on day 6 for the 2 patients with intact spleen, and 75 to 93 × 109/L and 60 to 84 × 109/L on days 3 to 5 for the splenectomized patients (supplemental Table 2). Hemoglobin levels decreased slightly during mobilization and leukapheresis (from 10.3-11.3 to 9.2-10.6 g/dL). The harvested CD34+ cell dose ranged from 8 to 12 × 106 /kg in 4 subjects who completed both leukaphereses (supplemental Table 2). One patient did not complete the second leukapheresis due to anxiety.
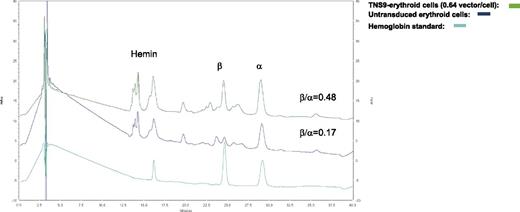
In prevalidation studies, we determined that the highest transduction efficiency utilizing cGMP TNS9.3.55 vector was obtained by performing 2 transduction cycles in presence of 100 ng/mL of thrombopoietin, human Flt3-L, stem cell factor, and 20 ng/mL IL-3, yielding a mean vector copy number (VCN) of 0.2 to 1.5 (unpublished data). Utilizing patient CD34+ HPCs, we performed 3 validation runs under these optimized conditions, which yielded an average VC/cell of 0.53 ± 0.13 (Table 1). The percentage of formulated CD34+ HPCs was 98.8% ± 1.0% and the fold expansion was 1.5 ± 0.25 (Table 1). The transduced cell dose obtained in validations 2 and 3 was 2 and 10 × 106 CD34+ cells/kg, respectively, and met the release criteria of 0.2 to 2.0 VC/cell. A mean VCN of 1 was observed in individual hematopoietic colonies (supplemental Figure 1A). The TNS9.3.55-transduced CD34+ HPCs gave similar numbers of colony forming units (CFUs) to untransduced CD34+ cells (day 0) (supplemental Table 4). Prior to transduction, HPLC analyses showed reduced β/α-globin chain ratios of 0.25, 0.17, and 0.32, which increased to 0.36, 0.48, and 0.54, respectively (Figure 1), with corresponding VCNs of 0.39, 0.64, and 0.63. The increased β/α ratio normalized to VCN was thus consistent between these 3 thalassemic patients (0.28, 0.48, and 0.35, respectively) and comparable to that measured in healthy donors (0.33-0.48 per endogenous β-globin gene copy). The level of β-chain expression derived from TNS9.3.55 is therefore 73% to 100% of normal hemizygous β-globin output.
Characterization of end of production CD34+ cells transduced with TNS9.3.55 vector
| . | No. cells transduced . | Avg VCN liquid EC . | Frequency vector +ve CFUs (%) . | % CD34 d 3 . | Fold expansion d 0-d 3 . | No. CD34+ cells frozen . | CD34+ cells/kg . |
|---|---|---|---|---|---|---|---|
| Validation No. | |||||||
| 1 | 12.5 × 106 | 0.39 | 12.7 | 99.8 | 1.2 | 26 × 106 | NA |
| 2 | 300 × 106 | 0.63 | 30.0 | 98.0 | 1.5 | 500 × 106 | 10.3 × 106 |
| 3 | 54 × 106 | 0.58 | 23.8 | 98.7 | 1.7 | 106 × 106 | 2.1 × 106 |
| Mean ± SD | 0.53 ± 0.13 | 22.2 ± 8.8 | 98.8 ± 1.0 | 1.5 ± 0.25 | |||
| 2 (+23 mo) | NA | 0.56 | 12.7 | 99.0 | NA | NA | NA |
| . | No. cells transduced . | Avg VCN liquid EC . | Frequency vector +ve CFUs (%) . | % CD34 d 3 . | Fold expansion d 0-d 3 . | No. CD34+ cells frozen . | CD34+ cells/kg . |
|---|---|---|---|---|---|---|---|
| Validation No. | |||||||
| 1 | 12.5 × 106 | 0.39 | 12.7 | 99.8 | 1.2 | 26 × 106 | NA |
| 2 | 300 × 106 | 0.63 | 30.0 | 98.0 | 1.5 | 500 × 106 | 10.3 × 106 |
| 3 | 54 × 106 | 0.58 | 23.8 | 98.7 | 1.7 | 106 × 106 | 2.1 × 106 |
| Mean ± SD | 0.53 ± 0.13 | 22.2 ± 8.8 | 98.8 ± 1.0 | 1.5 ± 0.25 | |||
| 2 (+23 mo) | NA | 0.56 | 12.7 | 99.0 | NA | NA | NA |
Selected CD34+ HPC were either used fresh upon storage at 4°C overnight (validations 2 and 3) or thawed (validation 1). In validation 1, GLP vector stocks were used; in validations 2 and 3, vector stocks manufactured under cGMP were used. Two rounds of transduction at a multiplicity of infection of 25 were performed in presence of 100 ng/mL of TPO, Flt3-L, SCF and 20 U/mL interleukin-3. Approximately 80 CFUs per validation run were screened for the TNS9.3.55 by quantitative polymerase chain reaction. Avg, average. CFU, colony forming unit; EC, erythroid culture (d 14 bulk); NA, not applicable; SCF, stem cell factor; TPO, thrombopoietin.
Restoration of β-globin chain synthesis in patient hematopoietic cells.
We tested the engraftment of G-CSF mobilized transduced CD34+ HPCs derived from thalassemic patients in NSG mice. At 3.5 to 7 months posttransplantation, up to 54% huCD45+ cells were present in the recipient’s bone marrow (BM), which contained both lymphoid and myeloid lineage cells (supplemental Table 5). Importantly, the TNS9.3.55 vector remained present in the range of 0.17 to 0.6 VC/cell, as well as in individual BFU-Es retrieved from the same BM 6 to 7 months after transplantation, which showed 31% to 36% of the colonies harboring on average 1 copy of the vector (Table 2 and supplemental Figures 1A-D). Significantly, the mean VCN in the engrafted BM cells was 36% to 100% of that measured in the infused bulk CD34+ (range, 0.38-1.4 VC/cell) (Table 3), averaging 69% of input VCN after 3 to 7 months (n = 17 mice) (Tables 2 and 3). Additionally the level of β-chain expression derived from the vector in the BFU-Es retrieved from the BM 7 months postengraftment is 78% of normal hemizygous β-globin output (Table 2). Altogether, these results demonstrate robust and persisting globin gene marking and gene expression in engrafted CD34+ cell progeny from thalassemic patients, at a level similar to or only moderately lower than that measured in the CD34+ cell-infused product.
Long-term engraftment in vivo in NOD SCID mice
| Experiment . | No. mice (mo post-BMT) . | No. CD34+/mouse . | % huCD45 in BM . | Avg VCN bulk BM . | Frequency vector +ve BFU-E/BM (%) . | β/α** Expression BFU-E/BM (%) . |
|---|---|---|---|---|---|---|
| Postinfusion (in vivo) | ||||||
| 1 | 1 (6) | 5.0 × 105 | 54 | 0.17 | 36.0 | ND |
| 2 | 6 (7) | 1.8 × 106 | 13.6 ± 9.8 | 0.50 ± 0.3 | 31.0 | 77.8 ± 55 |
| 3 | 3 (6) | 2.0 × 106 | 0.8 ± 0.2 | 0.54 ± 0.2 | ND | ND |
| 4 | 3 (6) | 6.7 × 105 | 2.5 ± 2.6 | 0.60 ± 0.9 | ND | ND |
| 5 | 4 (3) | 1.6 × 106 | 3.2 ± 4.6 | 0.60 ± 0.3 | ND | ND |
| Experiment . | No. mice (mo post-BMT) . | No. CD34+/mouse . | % huCD45 in BM . | Avg VCN bulk BM . | Frequency vector +ve BFU-E/BM (%) . | β/α** Expression BFU-E/BM (%) . |
|---|---|---|---|---|---|---|
| Postinfusion (in vivo) | ||||||
| 1 | 1 (6) | 5.0 × 105 | 54 | 0.17 | 36.0 | ND |
| 2 | 6 (7) | 1.8 × 106 | 13.6 ± 9.8 | 0.50 ± 0.3 | 31.0 | 77.8 ± 55 |
| 3 | 3 (6) | 2.0 × 106 | 0.8 ± 0.2 | 0.54 ± 0.2 | ND | ND |
| 4 | 3 (6) | 6.7 × 105 | 2.5 ± 2.6 | 0.60 ± 0.9 | ND | ND |
| 5 | 4 (3) | 1.6 × 106 | 3.2 ± 4.6 | 0.60 ± 0.3 | ND | ND |
Mice were infused with 5.0e5 to 2.0e6 transduced CD34+ HPCs. The average VCN and the frequency of BFU-E colonies positive for the vector were determined as well as the level of β/α chain expression in the BFU-Es derived from the BM of engrafted mice (BFU-E/BM β/α** expression was measured in 2 independent pools of transduced BFU-Es and averaged. Untransduced pool 1, n = 30; transduced with TNS9.3.55 pool 1, n = 32; average VCN = 1.2. Untransduced pool 2, n = 30; transduced with TNS9.3.55 pool 2, n = 30; average VCN = 1.1. Experiments 4 and 5 were conducted with TNS9.3.55-transduced CD34+ cells derived from validation 2 and validation 3, respectively. CFU-GM, granulocyte-macrophage CFU; ND, not done; +ve, positive.
Characteristics of EC and BFU-E derived from transduced CD34+ cells cultured in vitro
| Experiment . | Avg VCN liquid EC . | Frequency vector +ve BFU-E (%) . | β/α* Expression BFU-E (%) . |
|---|---|---|---|
| Preinfusion (in vitro) | |||
| 1 | 0.38 | 21.2 | ND |
| 2 | 1.40 | 23.0 | 50 ± 13.7 |
| 3 | 0.74 | 30.4 | ND |
| 4 | 0.63 | 34.8 | ND |
| 5 | 0.58 | 30.0 | ND |
| Experiment . | Avg VCN liquid EC . | Frequency vector +ve BFU-E (%) . | β/α* Expression BFU-E (%) . |
|---|---|---|---|
| Preinfusion (in vitro) | |||
| 1 | 0.38 | 21.2 | ND |
| 2 | 1.40 | 23.0 | 50 ± 13.7 |
| 3 | 0.74 | 30.4 | ND |
| 4 | 0.63 | 34.8 | ND |
| 5 | 0.58 | 30.0 | ND |
The average VCN in liquid EC and the frequency of BFU-E colonies positive for the vector were determined in 5 independent experiments. β/α* expression was measured in pooled BFU-Es: untransduced pool, n = 30; transduced with TNS9.3.55 n = 30; average VCN = 1.6. ND, not done.
The safe collection of HPCs in sufficient numbers and their efficient transduction are 2 essential prerequisites for the successful implementation of stem cell trials. These concerns are heightened in the case of β-thalassemia and sickle cell disease, which use complex tissue-specific vectors with lower titers than the more commonly used complementary DNA-encoding vectors.13,16,17 Furthermore, adult subjects will be treated on these trials, in contrast to the primarily pediatric subjects treated for metabolic disorders and severe immune deficiencies.18 Li et al19 previously administered G-CSF in 20 patients with β thalassemia aged 3- to 21-year-old, but no quantitative data on the yield or composition of the apheresis products were provided. Yannaki et al.20 mobilized 23 thalassemic adults with G-CSF with or without pretreatment with hydroxyurea. The CD34 cell yield was 3.8 to 9.4 × 106 CD34+ cells/kg in nonsplenectomized patients, but only 0.84 to 4.5 ×106 CD34+ cells/kg in splenectomized subjects. Our series comprised 5 heavily transfused thalassemic subjects including splenectomized and nonsplenectomized patients, none of which were pretreated with hydroxyurea. We obtained 8 to 12 × 106 CD34+ cells/kg for the 4 evaluable patients. The effect of adding plerixafor to this regimen warrants further exploration, as it could potentially reduce the apheresis to a single collection,20 and requires further transduction validation. CD34+ cell collection in patients with sickle cell disease, in whom G-CSF is poorly tolerated, remains an unresolved problem.21
Unlike other blood and metabolic disorders, in which achieving therapeutic transduction levels has been highly successful, globin gene transfer has long been a major obstacle due to low vector titers.12,13 Previous studies analyzing globin gene transfer in CD34+ HPCs were not performed under cGMP conditions or at large scale.22,23 In a clinical case report, peripheral blood marking levels were initially low before a single clone expanded and eventually compensated for overall low transduction efficiency.24 Here, we demonstrate for the first time that we can achieve globin gene transfer in thalassemic G-CSF mobilized HPCs in the range of 0.4 to 0.6 VC/cell under cGMP conditions. We and others previously estimated the minimal stable gene marking in peripheral blood cells required for a therapeutic effect to be on the order of 10%.25 However, the relationship between the VCN measured in transduced, bulk CD34+ cells, used as a release criterion, and the ultimate marking level in the recipient’s peripheral blood cells, is complex and difficult to predict. Importantly, we found that the VCN measured in the BM of mice engrafted with transduced CD34+ was 36% to 100% of the mean VCN determined in bulk CD34+ cell-derived erythroid cultures. Our validation studies thus bode favorably for reaching the 10% threshold level of gene marking in adult thalassemic subjects when the average VCN in transduced CD34 cells exceeds 0.2. Finally, we found normalized expression levels of vector-encoded human β-globin to approximate normal hemizygous expression, supporting our estimates that 1 vector copy/cell of TNS9.3.55 will effectively treat β+ thalassemia and may be sufficient in β0-thalassemia.
In summary, we demonstrate that CD34+ HPCs from adult thalassemic patients can be safely collected and effectively transduced at large scale in clinically relevant manner. These data provide the basis for a clinical trial, which was registered at ClinicalTrials.gov as #NCT01639690.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Lilian Reich and Joann Tonon for their assistance with the aphareses, Dr Chris Ballas for assistance with vector production, and the patients for their participation in the mobilization study.
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute P01 HL053750 (M.S. and I.R.) and National Cancer Institute P30 CA08748 (I.R.), the Stavros Niarchos Foundation, the Cooley’s Anemia Foundation, the Cooley's Anemia International, the Leonardo Giambrone Foundation, the Piera Coutino Foundation, Errant Gene Therapeutics, LLC, and the Department of Pediatrics at Memorial Sloan-Kettering Cancer Center.
Authorship
Contribution: F.B., I.R., and M.S. designed the research plan; F.B., M.S., G.H., and I.R. designed the clinical studies; X.W., C.T., J.Q., L.F., and G.K. performed research; C.T., J.Q., L.F., G.K., and S.B. contributed to quality control testing; F.B., P.G., S.E.P., and A.M. enrolled patients in the study and took care of the patients; G.H. oversaw statistical analyses; I.R. directed cell processing and molecular monitoring studies; and F.B., X.W., I.R., and M.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Isabelle Rivière, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Box 182, New York, NY 10065; e-mail: rivierei@mskcc.org.
References
Author notes
F.B and X.W. contributed equally to this study.