Key Points
For good immune reconstitution and fewer viral reactivations, thymoglobulin should be omitted in cord blood transplants.
Because omission of thymoglobulin is associated with higher acute GVHD rates, further improvement of outcome may require individualized dosing.
Abstract
In vivo T-cell depletion might contribute to the delayed immune reconstitution observed after unrelated umbilical cord blood transplantation (UCBT). We studied the impact of early, late, and no antithymocyte globulin (ATG) on immune reconstitution and outcome. One hundred twenty seven children receiving UCBT in London or Utrecht were divided into 3 groups: early ATG (days −9 to −5; n = 33), late ATG (days −5 to 0; n = 48), and no ATG (n = 46). The no-ATG group received mycophenolate mofetile + cyclosporin A as graft-versus-host disease (GVHD) prophylaxis, while the ATG groups received cyclosporin A + prednisone. End points studied were survival, immune recovery, infections, and GVHD. The probability of survival was similar in all groups: no ATG, 71% ± 8%; early ATG, 68% ± 9%; and late ATG, 61% ± 7%. CD3+, CD4+, and CD4+-naive T-cell counts were significantly higher (P < .001) in the no-ATG group at 1, 2, 3, 6, and 12 months post-UCBT. In the no-ATG group, significantly fewer viral reactivations (P = .021) were noted. A higher probability of severe acute GVHD (aGVHD; 31%) was found in the no-ATG group compared with 18% (P = .018) for early-ATG and 5% (P < .001) for late-ATG groups. This was not associated with more chronic GVHD (cGVHD).
Introduction
Unrelated umbilical cord blood (UCB) is increasingly used as an alternative donor stem cell source for treating patients with leukemia, inborn errors of metabolism, immune deficiencies, and bone marrow failure syndromes. The use of UCB donors has important advantages, such as (1) prompt availability (HLA typed and banked); (2) less stringent HLA matching criteria, with a donor being available for >95% of the patients1 ; and (3) lower probability of graft-versus-host disease (GVHD), while maintaining a powerful graft-versus-leukemia effect.2-4 However, the use of UCB is also associated with a prolonged neutropenic period and higher rates of graft failure, particularly for UCB units with low cell numbers (<3 × 107 total nucleated cells per kilogram). Moreover, compared with matched sibling and matched unrelated donor hematopoietic cell transplantation (HCT), patients undergoing UCB transplantation (UCBT) experience a delayed T-cell reconstitution, with an increased risk of opportunistic infections during the first 3 months posttransplant.5-7 This is a significant concern in patients with ongoing bacterial or fungal infection at the time of transplantation and/or in patients with a history of multiple viral reactivations.
One explanation for delayed T-cell reconstitution in UCBT recipients may be the use of in vivo T-cell depletion with antithymocyte globulin (ATG; thymoglobulin) or alemtuzumab in the conditioning regimen. ATG doses of 10 mg/kg are usually given in the UCBT setting where, on average, 1 log fewer T cells are infused compared with a bone marrow donor graft. Importantly, in some pharmacokinetic (PK) studies, ATG can be detected in the recipient’s plasma up to 25 to 60 days after HCT.8,9 In recent years, some transplant centers have started to omit ATG in the UCB setting.10 However, comparative analyses have not been published previously. In line with our hypothesis, our group (Chiesa et al11 ) recently reported that in the absence of ATG, using cyclosporin A (CSA) and mycophenolate mofetile as GVHD prophylaxis, an excellent immune recovery can be observed following unrelated UCBT. From these data, we can conclude that the intrinsic immune recovery after UCBT in children can be very rapid and that early T-cell reconstitution in this context is thymus-independent and is driven by the peripheral expansion of naive cord blood lymphocytes infused with the graft. Moreover, it was observed that UCB-naive T lymphocytes appear to undergo a rapid conversion to a memory/effector phenotype in response to viral/antigenic stimuli within the first 2 months posttransplant12,13 and that a long-term diverse immune repertoire is achieved.14 Nevertheless, one drawback of omitting ATG prior to UCBT may be an increased incidence of GVHD or graft rejection which may have a negative impact on survival.
We report, to the best of our knowledge for the first time, the role and timing of ATG on immune reconstitution and clinical outcome in children undergoing unrelated UCBT. Despite the heterogeneity of this population, these data may provide better guidance for future decisions on the use of ATG (or other serotherapy) in UCBT protocols.
Patients and methods
Setting and study population
All patients who received an unrelated UCBT at either the University Medical Center (UMC) Utrecht or at Great Ormond Street Hospital (GOSH) in London were included in this study. Patients were divided into 3 groups: those who received ATG early (between days −9 and −5) in the conditioning regimen, those who received ATG late (between days −5 and 0) in the conditioning regimen, and those who received no ATG. Patients without ATG were transplanted in London between April 2008 and December 2011, while patients given ATG were transplanted in Utrecht between March 2004 and November 2011 (late ATG 2004-2009, early ATG 2009-2011). The ATG used was thymoglobulin at a total dose of 10 mg/kg, except for patients with active hemophagocytic lymphohistiocytosis who received a double dose and patients with a CD3+ T-cell count <300 × 106/L (before conditioning) who received a dose reduced by 50%. All immune reconstitution data were collected prospectively when patients were enrolled for HCT after written informed consent was obtained in accordance with the Declaration of Helsinki and approval was given by the institutional ethical committee for sample and data collection.
Transplantation details, conditioning, and supportive care
Characteristics of patients and transplants are summarized in Table 1. Children were conditioned with either a myeloablative conditioning (MAC) or a reduced-intensity conditioning (RIC) regimen prior to UCBT. For patients receiving intravenous busulfan-containing regimens in the UMC Utrecht, therapeutic drug monitoring was used to target to a myeloablative area under the curve of 85 to 95 mg⋅h/L.
Basic characteristics of patients
| Variable . | No ATG (n = 46) . | Early ATG (n = 33) . | Late ATG (n = 48) . | P . |
|---|---|---|---|---|
| Sex | N/S | |||
| Male | 28 | 20 | 22 | |
| Female | 18 | 13 | 26 | |
| Diagnosis | ||||
| Malignancy | 21 | 13 | 16 | .001 |
| Immunodeficiency (SCID) | 24 (17) | 10 (2) | 12 (2) | |
| Bone marrow failure | 0 | 1 | 3 | |
| Metabolic disease | 1 | 8 | 17 | |
| Refractory autoimmunity | 0 | 1 | 0 | |
| Age | .006 | |||
| Median | 1.8 | 5.5 | 2.3 | |
| Range | 0.1-12.2 | 0.1-22.7 | 0.2-21.2 | |
| Stem cell transplantation | N/S | |||
| First | 42 | 32 | 45 | |
| Second | 4 | 1 | 2 | |
| Third | 0 | 0 | 1 | |
| Conditioning regimen | ||||
| Myeloablative conditioning | 29 | 31 | 45 | <.001 |
| BU/CY | 1 | 24 | ||
| BU 85-95/FLU 160/(Clo) | 2 | 27 (+2) | 8 | |
| Treo 42/FLU 150 | 10 | 0 | ||
| Treo 42/CY 120 | 1 | |||
| BEAM | 1 | |||
| Total-body irradiation–based (12 to 14.4 Gy) | 6 | 2 | 6 | |
| BU 90/CY/VP-16 (TDM) | 3 | |||
| BU 90/CY 120/Mel 140 | 8 | 1 | ||
| Oral BU 16/CY 120 | 3 | |||
| Reduced-intensity conditioning | 16 | 2 | 3 | |
| Treo 36/FLU 150 | 10 | |||
| Treo 36/CY 200 | 6 | |||
| BU 3/8; CY 1/5; FLU 3/8 | 1 | 1 | ||
| BU 3/8; FLU 3/8 | 1 | 1 | ||
| FLU/CY 1/5 | 1 | |||
| No conditioning | 1 | 0 | 0 | |
| rATG Genzyme 10 mg/kg | 0 | 30 | 43 | |
| rATG Genzyme 5 mg/kg | 0 | 2 | 3 | |
| rATG Genzyme 20 mg/kg (HLH) | 0 | 1 | 2 | |
| UCB cell doses | ||||
| Nucleated cells per kilogram × 107 | .055 | |||
| Median | 8.1 | 5.8 | 7.3 | |
| Range | 2.6-17.7 | 0.8-33.2 | 0.8-28.0 | |
| CD34+ cells per kilogram × 105 | <.001 | |||
| Median | 3.4 | 1.4 | 2.2 | |
| Range | 0.4-29.6 | 0.2-9.5 | 0.2-9.7 | |
| HLA match (of 6 alleles) | ||||
| 6/6 | 18 | 18 | 13 | N/S |
| 5/6 | 25 | 13 | 28 | |
| 4/6 | 3 | 2 | 7 | |
| Number of UCB infused | ||||
| Single cord blood unit | 42 | 30 | 47 | |
| Double cord blood unit | 4 | 3 | 1 |
| Variable . | No ATG (n = 46) . | Early ATG (n = 33) . | Late ATG (n = 48) . | P . |
|---|---|---|---|---|
| Sex | N/S | |||
| Male | 28 | 20 | 22 | |
| Female | 18 | 13 | 26 | |
| Diagnosis | ||||
| Malignancy | 21 | 13 | 16 | .001 |
| Immunodeficiency (SCID) | 24 (17) | 10 (2) | 12 (2) | |
| Bone marrow failure | 0 | 1 | 3 | |
| Metabolic disease | 1 | 8 | 17 | |
| Refractory autoimmunity | 0 | 1 | 0 | |
| Age | .006 | |||
| Median | 1.8 | 5.5 | 2.3 | |
| Range | 0.1-12.2 | 0.1-22.7 | 0.2-21.2 | |
| Stem cell transplantation | N/S | |||
| First | 42 | 32 | 45 | |
| Second | 4 | 1 | 2 | |
| Third | 0 | 0 | 1 | |
| Conditioning regimen | ||||
| Myeloablative conditioning | 29 | 31 | 45 | <.001 |
| BU/CY | 1 | 24 | ||
| BU 85-95/FLU 160/(Clo) | 2 | 27 (+2) | 8 | |
| Treo 42/FLU 150 | 10 | 0 | ||
| Treo 42/CY 120 | 1 | |||
| BEAM | 1 | |||
| Total-body irradiation–based (12 to 14.4 Gy) | 6 | 2 | 6 | |
| BU 90/CY/VP-16 (TDM) | 3 | |||
| BU 90/CY 120/Mel 140 | 8 | 1 | ||
| Oral BU 16/CY 120 | 3 | |||
| Reduced-intensity conditioning | 16 | 2 | 3 | |
| Treo 36/FLU 150 | 10 | |||
| Treo 36/CY 200 | 6 | |||
| BU 3/8; CY 1/5; FLU 3/8 | 1 | 1 | ||
| BU 3/8; FLU 3/8 | 1 | 1 | ||
| FLU/CY 1/5 | 1 | |||
| No conditioning | 1 | 0 | 0 | |
| rATG Genzyme 10 mg/kg | 0 | 30 | 43 | |
| rATG Genzyme 5 mg/kg | 0 | 2 | 3 | |
| rATG Genzyme 20 mg/kg (HLH) | 0 | 1 | 2 | |
| UCB cell doses | ||||
| Nucleated cells per kilogram × 107 | .055 | |||
| Median | 8.1 | 5.8 | 7.3 | |
| Range | 2.6-17.7 | 0.8-33.2 | 0.8-28.0 | |
| CD34+ cells per kilogram × 105 | <.001 | |||
| Median | 3.4 | 1.4 | 2.2 | |
| Range | 0.4-29.6 | 0.2-9.5 | 0.2-9.7 | |
| HLA match (of 6 alleles) | ||||
| 6/6 | 18 | 18 | 13 | N/S |
| 5/6 | 25 | 13 | 28 | |
| 4/6 | 3 | 2 | 7 | |
| Number of UCB infused | ||||
| Single cord blood unit | 42 | 30 | 47 | |
| Double cord blood unit | 4 | 3 | 1 |
Nonparametric tests were performed. P < .05 is considered significant.
BEAM, carmustine, etoposide, cytarabine, melphalan; BU, busulfan; CY, cyclophosphamide; CLO, clofarabine; FLU, fludarabine; HLH, hemophagocytic lymphohistiocytosis; Mel, melphalan; N/S, nonsignificant; rATG, rabbit ATG; SCID, severe combined immunodeficiency; TDM, therapeutic drug monitoring; Treo, treosulfan; VP-16, etoposide.
GVHD prophylaxis consisted of CSA supplemented with prednisolone 1 mg/kg at the UMC Utrecht or with mycophenolate mofetile 15 mg/kg three times a day at GOSH. CSA was administered from 2 or 3 days pre-UCBT, with a targeted trough level of 150 to 250 ng/mL.
All patients received recombinant human granulocyte colony-stimulating factor 5 μg/kg per day at GOSH from day +14 until neutrophil recovery (1 × 109/L) and 10 μg/kg per day at the UMC Utrecht from day +7 until neutrophil recovery (2 × 109/L).
Antimicrobial prophylaxis consisted of ciprofloxacin (from start of the conditioning regimen until neutrophil recovery), acyclovir (from start of the conditioning regimen until CD4+ T-cell recovery for varicella-zoster virus–positive patients), and itraconazole (GOSH) or voriconazole (UMC Utrecht) from the start of conditioning until neutrophil recovery, and no steroid treatment. Streptococcus viridans prophylaxis consisted of cefazolin (from day 0) at UMC Utrecht, and patients treated in London received vancomycin prophylaxis (from day +1) from year 2010. Prevention of Pneumocystis jirovecii pneumonia included cotrimoxazole from myeloid recovery, once every three weeks until at least 6 months (UMC Utrecht) or a CD4+ T-cell count >300 × 106/L (GOSH) and absence of chronic GVHD (cGVHD)/immunosuppressive treatment.
Donor selection and processing of the graft
UCB units were obtained from national (or international) cord blood banks. UCB units were required to be HLA matched to the patient at 4/6 or greater for HLA-A, HLA-B, and HLA-DRB1 antigens (HLA-A and HLA-B serologic typing and HLA-DRB1 high-resolution allele typing). The minimum total nucleated cell dose required for the transplant was 2.5, 3, or 5 × 107 nucleated cells per kilogram in case of a 6/6, 5/6, or 4/6 HLA-matched UCB unit, respectively. Children who did not meet these criteria received a double UCBT: both CB units were required to be a 4/6 match with each other and the patient and have a minimal cumulative cell dose of 4 × 107 nucleated cells per kilogram.
In GOSH, prior to transplantation, full allelic typing of HLA-A, -B, -C, -DRB1, and -DQB1 antigens was performed on the cord sample and from April 2010, units with more than three allelic mismatches were not selected.
Post-UCBT follow-up
Engraftment and chimerism.
Myeloid recovery was defined as the first of 3 consecutive days with an absolute neutrophil count exceeding 0.5 × 109/L, and platelet recovery was defined as an unsupported platelet count of 20 × 109/L (thrombocyte blood count 20 × 109/L) and 50 × 109/L (thrombocyte blood count 50 × 109/L). Chimerism studies were performed by short tandem repeat variability on peripheral blood samples from the first signs of engraftment (lymphocyte count >0.4 × 109/L) and repeated every 2 to 4 weeks until >95% UCB chimerism was observed. Mixed chimerism was defined as the presence of >5% recipient DNA.
Immune reconstitution.
CD3+, CD4+, and CD4+-naive B-cell and natural killer (NK) –cell numbers were prospectively measured during follow-up at 1, 2, 3, 6, and 12 months post-UCBT for subset analysis. All patients were included in immune-reconstitution studies approved by the respective institutional review boards, and written informed consent was obtained from patients’ parents or legal guardians in each case according to the Declaration of Helsinki. Cell counts were performed by using an automated cell counter. A reagent containing CD3− fluorescein isothiocyanate (FITC), CD45− peridinin chlorophyll protein (FITC PerCP), CD19− allophycocyanin (FITC APC) or CD3− FITC, CD8− phycoerythrin (FITC PE), CD45− peridinin chlorophyll protein (FITC PerCP), or CD4− allophycocyanin (FITC APC) (Multitest, BD) was added to a fluorescence-activated cell sorter tube containing a known quantity of beads, followed by 100 μL of EDTA (ethylenediaminetetraacetic acid) anticoagulated whole blood and incubated for 10 minutes at room temperature. Red bloods cells were subsequently lysed for 10 minutes with fluorescence-activated cell sorter lysing solution (BD). Samples were acquired by using a FACSCalibur flow cytometer and were analyzed by using BD software. The following lymphocyte subsets were counted: CD3+ T lymphocytes, CD3+CD4+ and CD3+CD8+ T lymphocytes, CD19+ B lymphocytes, CD3−/CD16+CD56+ NK lymphocytes, CD45RA+CD27+-naive T lymphocytes, CD45RA−CD27+ memory T lymphocytes, and CD45RA+CD27− effector T lymphocytes.
Antimicrobial monitoring.
As standard of care, viral (Epstein-Barr virus [EBV], cytomegalovirus [CMV], adenovirus) DNA loads were weekly monitored in the peripheral blood by using quantitative polymerase chain reaction.
Remission status.
In GOSH, bone marrow studies were standard after engraftment. In the UMC Utrecht, they were not performed routinely but only in case of poor engraftment or if there was evidence of disease relapse in the peripheral blood. Relapse was based on hematologic, immunologic, and pathologic confirmation of disease recurrence.
Posttransplant complications were defined similarly in both centers. Death without underlying disease progression was considered transplant related. Toxicity was scored according to National Cancer Institute Common Toxicity Criteria. The diagnosis of acute GVHD (aGVHD) was made clinically and confirmed pathologically with skin, mucosal, or liver biopsy whenever possible. Grading of aGVHD was performed according to the Seattle criteria with the caveat of late aGVHD to occur after day 100, as described by the National Institutes of Health consensus guidelines.15,16 cGVHD was defined according to National Institutes of Health guidelines.
The definition of noninfectious lung injury includes idiopathic pneumonia syndrome and bronchiolitis obliterans syndrome; this definition was used only for those in whom no infectious cause could be established (by respiratory viral polymerase chain reaction and bacterial cultures of tracheal secretions, imaging, aspiration of localized lesions identified with imaging, and/or postmortem pathology results, if available). Idiopathic pneumonia syndrome was defined as acute bilateral infiltrates with cough, dyspnea, and hypoxemia in the absence of infection. Bronchiolitis obliterans syndrome was defined as typical high-resolution computed tomography changes such as bronchial wall thickening, air trapping, and mosaic attenuation and, if pulmonary function testing could be performed, as a decrease of ≥20% in forced expiratory volume at 1 second, again in the absence of signs of infection.
End points
Primary end points were overall survival (defined as survival from UCBT to death or last contact), event-free survival (EFS; defined as survival time from the hematopoietic stem cell transplantation to relapse of malignant disease, death, or graft failure), nonengraftment (defined as a lack of neutrophil recovery to ≥0.5 × 109/L), secondary graft failure (transient engraftment followed by a progressive decline of donor cells after transplantation with a requirement for a second transplant), relapse of malignant disease, and nonrelapse mortality (NRM; death not due to underlying malignant disease). Secondary end points were immune reconstitution as measured by absolute numbers of CD3+ and CD4+ T cells at 1, 2, 3, 6, and 12 months post-UCBT (early immune reconstitution was defined as a CD3+ T-cell count of at least 300 × 106/L within 2 months post-UCBT); aGVHD grade II to IV and III to IV, cGVHD, viral reactivation of CMV, EBV, or adenovirus, multiple viral infections, and opportunistic infection.
Statistical analyses
The data were retrospectively analyzed. Differences in patients’ characteristics and in immune reconstitution parameters were assessed by using Kruskal-Wallis tests for continuous data and χ2 tests for categorical data. The outcome parameters used for comparison between the three groups were EFS, relapse, NRM, immune recovery, graft failure, and aGVHD and cGVHD incidence.
To analyze risk factors for outcomes in the whole cohort, we considered patient-related factors (median age at transplant, gender), the disease (malignant/nonmalignant), use of ATG (plus timing), donor factors (HLA disparity and median collected and infused total nucleated cell and CD34+ cell doses), stem cell transplant number (first, second, third), and conditioning regimen (MAC or RIC). Analyses of the associations between the various time-dependent variables (the various main survival end points and aGVHD) were performed by using Cox proportional hazards models. Univariate predictors of outcome that were statistically significant (P ≤ .10) were selected for multivariate Cox proportional hazards models. Results are expressed as hazard ratios (HRs) and their corresponding 95% confidence intervals (95% CIs).
For analyses of dichotomous outcome parameters, univariate and multivariate logistic regression analyses were used. Dichotomous outcomes (eg, viral reactivation: yes/no) were used as dependent variables, and predictors were used as independent variables. Univariate predictors of outcome that were statistically significant (P ≤ .10) were selected for multivariate logistic regression analysis. Results are expressed as odds ratios (ORs) and their corresponding 95% CIs. CIs not including 1 (P ≤ .05) were considered statistically significant. Statistical analysis was performed by using SPSS version 19.0.
Results
Patients, donors, and transplant characteristics
A total of 127 patients were included; 48 in the late-ATG group, 33 in the early-ATG group, and 46 in the no-ATG group (Table 1). No difference in ATG dose was found between the two ATG groups. RIC regimens were more common in the no-ATG group.
Indication for UCBT was different among the treatment groups: there was a larger proportion of children with primary immunodeficiencies in the no-ATG group and a higher proportion of metabolic diseases in the late-ATG and early-ATG groups (Table 1). This difference resulted in a lower median age at transplantation (P = .006) and a higher dose of UCB CD34+ cells per kilogram in the no-ATG group (Table 1). Although cord blood selection rules differed between the two centers, HLA disparity ended up not being different between centers (P = .49).
Engraftment and survival
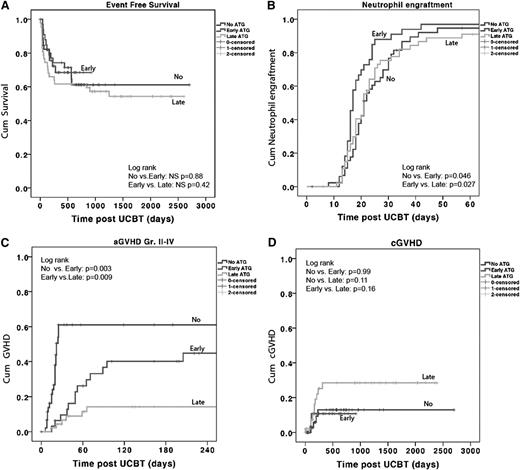
The CI for neutrophil engraftment within 60 days post-UCBT was 95% ± 4% for the no-ATG group, 97% ± 3% for the early-ATG group, and 90% ± 4% for the late-ATG group. A more rapid myeloid and platelet recovery was observed in the early ATG group (Table 1; Figure 1B); the median time to neutrophil recovery was 21 days in the no-ATG group as opposed to 17 days in the early-ATG group (P = .01) and 21 days (not significant) in the late-ATG group. Nonengraftment rates were low, with no statistically significant difference among the 3 groups (no ATG, 4%; early ATG, 3%; and late ATG, 10%; P = not significant). Secondary graft failure rates also were not statistically different: 2%, 3%, and 11%, respectively.
ATG in cord blood transplants does not influence EFS and engraftment but does lead to more aGVHD. Kaplan-Meier survival curve and Cox proportional hazards analysis of the (A) probability of EFS, (B) probability of neutrophil engraftment: neutrophil count of 0.5 × 109/L for at least 3 days in the first 60 days after stem cell transplant (SCT), (C) probability of aGVHD grade II to IV, and (D) probability of cGVHD. P < .05 is considered significant. Patients represented as having received no ATG, received ATG early, or received ATG late. NS, nonsignificant.
ATG in cord blood transplants does not influence EFS and engraftment but does lead to more aGVHD. Kaplan-Meier survival curve and Cox proportional hazards analysis of the (A) probability of EFS, (B) probability of neutrophil engraftment: neutrophil count of 0.5 × 109/L for at least 3 days in the first 60 days after stem cell transplant (SCT), (C) probability of aGVHD grade II to IV, and (D) probability of cGVHD. P < .05 is considered significant. Patients represented as having received no ATG, received ATG early, or received ATG late. NS, nonsignificant.
The 3-year estimated probability of overall survival was similar in the 3 groups (no ATG, 71% ± 8%; early ATG, 68% ± 9%; and late ATG, 61% ± 7%). NRM, relapse rate, and EFS were also not significantly different. The 3-year estimate of the probability of EFS was 61% ± 8% for the no-ATG group, 68% ± 8% for the early-ATG group, and 55% ± 7% for the late-ATG group (Figure 1A).
In multivariate analysis, only the use of a mismatched 4/6 or 5/6 UCB unit (HR, 4.2; 95% CI, 1.1 to 15.8; P = .033) was found to be a predictor for lower EFS. The use of ATG was not. For NRM, none of the variables were found to be significant predictors.
Immune reconstitution
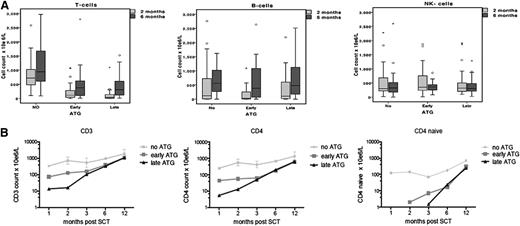
Immune reconstitution data are available for 85% to 88% of the time points of 1, 2, 3, 6, and 12 months post-UCBT. By using multivariate analysis, the only predictor for early immune reconstitution after UCBT was the omission of ATG from the conditioning regimen (no ATG: OR, 57; 95% CI, 12 to 256; P < .001). We found that CD3+, CD4+, and CD4+-naive T-cell numbers were 1 log higher in the no-ATG group compared with the early-ATG and late-ATG groups at 1, 2, 3, 6, and 12 months post-UCBT (P < .001; Figure 2B). The median CD3+ T-cell count in the no-ATG group at 1, 2, 3, 6, and 12 months post-UCBT was 340 × 106/L, 720 × 106/L, 535 × 106/L, 940 × 106/L, and 1860 × 106/L, respectively (Figure 2B).
ATG affects post-SCT T-cell immune reconstitution. (A) Comparison of CD3+ cell numbers, B-cell numbers, and NK-cell numbers for the 3 groups at 2 months post-SCT and 6 months post-SCT. (B) Logarithmic evaluation of CD3+, CD4+, and CD4+-naive cell counts for time post-SCT (months).
ATG affects post-SCT T-cell immune reconstitution. (A) Comparison of CD3+ cell numbers, B-cell numbers, and NK-cell numbers for the 3 groups at 2 months post-SCT and 6 months post-SCT. (B) Logarithmic evaluation of CD3+, CD4+, and CD4+-naive cell counts for time post-SCT (months).
Interestingly, patients in the early-ATG group had significantly higher CD3+, CD4+, and CD4+-naive T-cell counts at 1 and 2 months post-UCBT when compared with patients in the late-ATG group (1 month, P = .02; 2 months, P = .036; 3 months, P = .13; Figure 2). There was no difference in NK- and B-cell recovery among the 3 groups: normal NK cell numbers were observed in all groups 1 month post-UCBT, and normal B cells were observed 2 months post-UCBT (Figure 2A).
Transplant-related complications
Viral reactivations.
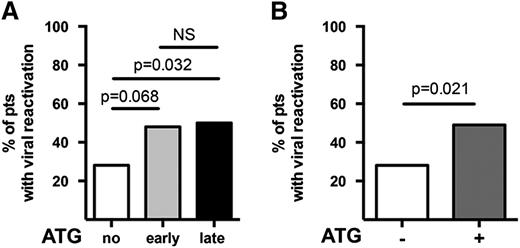
The no-ATG group showed a lower number of episodes of viral reactivations (P = .021) compared with patients who received ATG (Figure 3), while no difference was observed between the early-ATG and late-ATG groups. There was also a lower number of deaths from viral infections (P = .002) in the group with no ATG compared with the groups that received ATG. Specifically for EBV, preemptive therapy with rituximab 375 mg/m2 was administered to 10 patients (2 in the no-ATG group, 2 in the early-ATG group, and 6 in the late-ATG group). However, clinically apparent posttransplant lymphoproliferative disorder was diagnosed in only 1 additional patient who recovered after treatment and has good immune reconstitution and quality of life. Four of the 11 patients treated for EBV died. None of them died directly of EBV infection. One died of aGVHD, another of invasive fungal infection, and two of toxicity-related multiorgan failure and GVHD. All four were in the late-ATG group. The number of patients with opportunistic (including fungal) infections was not different for the 3 groups (P = .24).
Lower incidence of viral reactivation in cord blood transplants without ATG. The incidence of viral reactivation of adenovirus, CMV, or EBV is compared (A) for each of the 3 groups and (B) for groups with and without ATG. Mann-Whitney U tests have been performed. P < .05 is considered significant. NS, nonsignificant; pts, patients.
Lower incidence of viral reactivation in cord blood transplants without ATG. The incidence of viral reactivation of adenovirus, CMV, or EBV is compared (A) for each of the 3 groups and (B) for groups with and without ATG. Mann-Whitney U tests have been performed. P < .05 is considered significant. NS, nonsignificant; pts, patients.
GVHD and noninfectious lung injury
With multivariate analysis, both no-ATG (HR, 8.2; 95% CI, 3.2 to 22.0; P < .001) and early-ATG (OR, 3.9; 95% CI, 1.5 to 10.5; P = .005) groups were independent predictors for aGVHD as opposed to the late-ATG group.
A higher incidence of grade II to IV aGVHD (P = .003) and grade III to IV aGVHD (P = .018) was observed in the no-ATG group (Figure 1C) compared with the early-ATG group. Patients in the early-ATG group experienced a significantly higher incidence of grade II to IV aGVHD compared with patients in the late-ATG group (44% vs 14%; P = .009). However, no difference was found for grade III to IV aGVHD (18% vs 5%; P = .15) between the 2 ATG groups (data not shown). Despite the higher rate of aGVHD in the no-ATG and early-ATG groups, the incidence of cGVHD was not statistically different among the 3 groups: 12% ± 6% in the no-ATG group, 11% ± 6% in the early-ATG group, and 28% ± 8% in the late-ATG group (Figure 1D).
The lowest incidence of noninfectious lung injury was found in the early-ATG group (17 ± 7%), but this was not significantly different from the other 2 groups (no-ATG group, 37% ± 15% [P = .42]; late-ATG group, 27% ± 7% [P = .19]; data not shown).
Discussion
In this study, we demonstrate that the use of in vivo T-cell depletion prior to UCBT significantly affects early and late posttransplant T-cell reconstitution. It cannot be ruled out that differences between the HCT platforms in the two centers (eg, GVHD prophylaxis and conditioning regimen) contributed to the better immune reconstitution and higher GVHD rate observed in the no-ATG group. However, the hypothesis that ATG is important is supported by the finding that early rather than late administration of ATG also facilitated a better T-cell recovery within the first 2 months post-UCBT using the same transplant platform. Furthermore, a similar observation has also been done for double UCBT without ATG by Sauter et al,10 showing a steady immune recovery, a low infection-related mortality after day 120, but a higher GVHD risk compared with patients who received ATG prior to double UCBT.
In this article, we showed that children transplanted without ATG as part of their conditioning regimen experienced a remarkably quick CD3+ cell recovery soon after UCBT, with a median T-cell count of 720 × 106/L only 2 months after transplant. This is in contrast to those patients treated with early or late ATG in whom a median CD3+ T-cell count of <100 × 106/L was achieved at the same time point. The lymphocyte recovery was biased toward CD4+ as previously described,9,17 and the reason for this is currently unknown. Other studies have also shown a comparable pattern of poor early T-cell recovery after UCBT in the context of in vivo T-cell depletion.1,6,17-19
The improved immune reconstitution in the group not receiving ATG prior to transplantation was associated with a lower incidence of reactivation of EBV, CMV, and/or adenovirus. Various reports have shown that UCBT recipients are at increased risk of viral reactivation because of the delayed immune recovery,2-4,20-22 and early initiation of preemptive antiviral therapy is frequently used to prevent viral disease using a lower cutoff quadruplex DNA value in CB recipients.5,6,23,24 Although we did not find MAC or RIC regimens to be predictors in this cohort, others have found ATG used in association with RIC regimens to further increase the risk of viral reactivation and specifically the risk of EBV posttransplantation lymphoproliferative disorder (PTLD).25,26 Overall, EBV PTLD was easily manageable in this cohort with preemptive treatment. Only 1 patient developed PTLD, and she recovered completely.
The observed reduced incidence of viral reactivations in the no-ATG group is likely the result of not depleting the UCB graft of lymphocytes, which although naive, have the capacity of rapidly differentiating into the effector/memory phenotype in response to antigen stimulation, even early after UCBT.8,9,11,27 It could be speculated that an early specific immune reconstitution post-UCBT may also lead to a more powerful graft-versus-leukemia effect, as suggested in other studies.2,11,28-30 The number of patients with malignant disease in each group in this study was too small and disease type and conditioning were too heterogeneous to assess this.
In our pediatric population of children with mixed malignant and nonmalignant indications, ATG was not required for engraftment because the CIs of neutrophil engraftment at day 60 were similar between groups (Figure 1B). In contrast with others, we found HLA disparity to be a predictor for EFS but not cell dose. This can be explained by the strict cell dose rules that were applied and the low median weight of the studied cohort leading to a high median cell dose in this study of 7.3 × 107 (range, 0.8 to 33.2 × 107) nucleated cells per kilogram after thawing.
The clinical benefit of an early immune reconstitution in the no-ATG group has to be balanced against the higher incidence of moderate to severe aGVHD observed in this cohort. Interestingly, this was not associated with a higher incidence of cGVHD or NRM. Historically, UCBT has been associated with a lower incidence of aGVHD compared with matched unrelated donor HCT, as shown in various comparison studies. This study provides some perspective, showing that the incidence of aGVHD in UCBT is very much influenced by in vivo T-cell depletion. Rates of aGVHD reported in this study are higher than those previously published by other authors: for example, Verneris et al31 reported grade II to IV aGVHD rates of 29% for single UCBT and 48% for double UCBT.12,13,31 A more recent and large Eurocord study showed similar GVHD incidences in single and double UCBT.15,16,32 MacMillan et al33 reported that the omission of ATG prior to double UCBT did not influence mortality and led mainly to an increased incidence of grade II skin GVHD.10,33 The aGVHD rates in our cohort, however, may also be influenced by the selection of primary immunodeficiency patients in the no-ATG group. Such children may be more at risk for aGVHD because of ongoing infections and tissue inflammation. Importantly, however, a higher rate of aGVHD did not lead to a higher incidence of cGVHD, viral reactivations, or higher NRM.
In the most commonly used UCBT protocols, particularly with late administration of ATG, we most likely administered an overdose of ATG causing severe in vivo depletion of the graft, resulting in absent or very late immune reconstitution. In fact, there are centers that therefore use doses lower than 10 mg/kg in their conditioning regimen, especially in adults. It has recently been shown that, for adults using peripheral blood stem cells as the donor source in a RIC setting, ATG as high as 8 mg/kg pretransplant can have a deleterious influence, increasing the risk of relapse compared with the dose of 6 mg/kg.34
Potentially, various patient/donor-dependent variables such as weight, lymphocyte count prior to UCBT, and age influence ATG PK and pharmacodynamics (PD).35 Further improving outcome after UCBT may therefore require a more individualized approach to ATG timing and dose adapted to indication, immunologic status, and infectious comorbidities of the patient. This can possibly be achieved by implementing PK/PD modeling techniques for antibody agents such as ATG. In a joint project between two pediatric blood and bone marrow transplantation units in the Netherlands (Utrecht and Leiden), ATG PK/PD studies are currently being performed in more than 300 patients. This will be the basis for the development of a worldwide applicable dosing algorithm (eg, based on weight and age).8 Other more novel GVHD prevention strategies such as histone deacetylase inhibitors, proteasome inhibitors, antibodies targeting interleukin-21, vascular adhesion molecules, B-cell targeting, or regulatory T-cell and mesenchymal stem cell infusions may also be among the options for diminishing GVHD risk post-UCBT without ATG.36
An early specific and diverse immune reconstitution is crucial for a good outcome after transplantation, since relapse and NRM (due to aGVHD and viral reactivation) are the most important limiting factors.37 Currently, the improved immune reconstitution associated with lower viral reactivations, albeit at the cost of increased rates of aGVHD (but not cGVHD), suggests that omitting ATG in UCBT may be suitable for children treated for malignancy or children at risk of viral reactivation, while children with otherwise uncomplicated nonmalignant disorders may benefit from reduced aGVHD with inclusion of early ATG in the conditioning regimen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a clinical fellowship from the Dutch Cancer Society (C.L.).
Authorship
Contribution: C.A.L. and R.C. conducted the research, analyzed the data, and wrote the paper; P.J.A., K.R., K.C.G., and O.N. contributed to the data from the United Kingdom and the design of immune-reconstitution studies; C.E.G. and A.d.W. contributed to the data from Utrecht; M.B.B. was involved in the design of immune reconstitution studies, critical reviewing of the data, and collaboration between the centers; and P.V. and J.J.B. performed research, analyzed data, wrote the paper, and were involved in the design of immune-reconstitution studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Caroline A. Lindemans, UMC Utrecht, KC03.063.0, PO Box 85090, 3508 AB Utrecht, The Netherlands; e-mail: c.a.lindemans@umcutrecht.nl.
References
Author notes
C.A.L. and R.C. are joint first authors.
There is an Inside Blood commentary on this article in this issue.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal