Key Points
Complement factor H and von Willebrand factor colocalize in the Weibel-Palade bodies of endothelial cells and interact in normal plasma.
Formation of the complex enhances FH cofactor activity and VWF-mediated platelet aggregation.
Abstract
Vascular endothelial cells (ECs) link hemostasis, thrombosis, and complement. ECs synthesize both the clotting initiator von Willebrand factor (VWF) and the complement regulator factor H (FH). VWF is stored in EC Weibel-Palade bodies (WPBs), but the intracellular location of FH is not well defined. We found that FH colocalizes with VWF in WPBs of human umbilical vein ECs. Moreover, FH bound to VWF with an apparent nanomolar affinity and the complex was present in normal plasma. The binding of VWF to FH enhanced FH cofactor activity toward factor I–mediated downregulation of complement activation. Besides, this interaction inhibited ADAMTS13-mediated proteolysis of VWF and promoted platelet aggregation. Here, we describe a novel interaction between complement and hemostasis. The simultaneous secretion of VWF and FH by activated ECs may promote adhesion of platelets to endothelial injury sites to assure wound healing, simultaneously dampening the proinflammatory effect of complement to limit bystander tissue damage.
Introduction
Endothelial cells (ECs) represent an important interface between primary hemostasis, coagulation, and complement activation. During inflammation and endothelial injury, activated ECs release procoagulant and proinflammatory molecules, including multimeric von Willebrand factor (VWF) that is stored in the Weibel-Palade bodies (WPBs). In addition, ECs secrete complement molecules including factor H (FH).1-4 We investigated the colocalization of FH and VWF in the WPBs of human ECs. We further demonstrated the interaction between the 2 molecules and the relevance of this interaction for their respective functions.
Study design
Patient
Blood was collected, with informed consent provided according to the Declaration of Helsinki, during the normal follow-up of a type 3 von Willebrand disease (VWD) patient with combined heterozygous p.Gln1311X and p.Ser2079X mutations, 5 days after replacement therapy. Because the study did not require additional blood sampling, an approval from an ethics committee was not required under French law according to article L.1121-1 of the public health code.
FH binding to VWF by ELISA
VWF (Wilfactin, LFB, or recombinant VWF; Baxter) or polyclonal anti-VWF antibodies were coated on enzyme-linked immunosorbent assay (ELISA) plates (Nunc) and incubated with normal plasma, FH (Comptech), or recombinant FH fragments consisting of domains 1 to 4, 6 to 8, and 19 to 20.5,6 Binding of FH or fragments was revealed using a goat anti-human FH antiserum (Quidel).
Surface plasmon resonance
VWF was coupled to the CM5 biosensor chip using a Biacore 2000 (GE Healthcare). FH binding to both VWF-coated and uncoated flow cells was monitored. Data were analyzed using the BIAevaluation software after subtraction of the signal generated by the uncoated flow cell.
Immunoprecipitation of FH-VWF complex
Immunoglobulin G (IgG)-depleted plasma (by protein G adsorption) was incubated with CNBr-sepharose beads (Sigma-Aldrich) coated with anti-VWF IgG (Dako). Immunoprecipitated FH was detected by immunoblotting.
Immunostaining
Fixed and permeabilized human umbilical vein ECs (HUVECs) grown on coverslips were incubated with a pool of 3 biotinylated anti-FH antibodies: FH1 and FH2 (Quidel) and OX24 (AbDSerotec) and with a polyclonal anti-VWF antibody (Dako). Cells were washed and mounted in fluorescent mounting medium Fluoprep (bioMérieux). Images were acquired using an Axiovert 200M microscope (Zeiss) equipped with Apotome.
FH activity
FH cofactor activity was assessed using purified C3b, factor I (FI), and iC3b (Comptech). The reaction was stopped by adding reducing sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis sample buffer and analyzed by immunoblotting with a goat polyclonal anti-human C3 antibody (Calbiochem) and a rabbit anti-goat IgG–horseradish peroxidase (Southern Biotech).
VWF proteolysis by ADAMTS13
Results and discussion
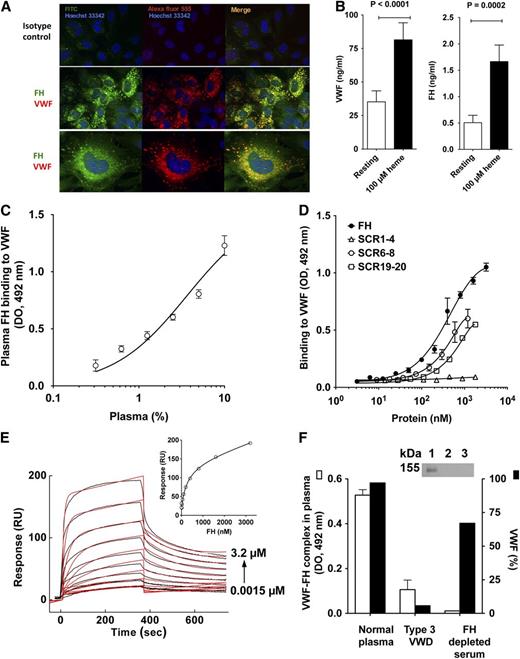
In the case of endothelial injury, complement and coagulation are simultaneously activated to assure wound healing and elimination of the aggressing agent. These cascades have to be tightly regulated to avoid overactivation, which would otherwise lead to tissue injury aggravation rather than healing. We investigated the respective localizations of complement regulator FH and VWF in resting HUVECs. When HUVECs were stained for FH and VWF, both molecules appeared colocalized in the WPB (Figure 1A). The detection of FH in the WPBs is in contrast with earlier work that used different detecting antibodies.4 It also contrasts with the absence of FH localization with VWF in the α-granules of platelets.9 Stimulation of HUVECs with heme10 resulted in 2.3-fold and 3.3-fold increases in VWF and FH concentration in culture supernatant, respectively (Figure 1B), suggesting that some of the FH found in the circulation originates from ECs. Both purified FH and FH in plasma interacted in a dose-dependent manner with immobilized recombinant (not shown) and plasma-derived VWF (Figure 1C-D), at physiological pH and ionic strength. VWF-FH complexes were detected in normal plasma by ELISA (Figure 1F) and by immunoprecipitation (Figure 1F inset). No complex was detected in the plasma of a type 3 VWD patient or in FH-depleted serum containing VWF, suggesting the specificity of the interaction. Using a polyclonal antibody that equally recognizes FH and all recombinant FH fragments (supplemental Figure 1), VWF was found to bind to both the recombinant FH fragments consisting of the short consensus repeat (SCR) domains 6 to 8 (SCR6-8) and domains 19 to 20 (SCR19-20) that are responsible for FH anchoring to the cell surface. No binding was observed to regulatory N-terminal domains 1-4 (SCR1-4) of FH that are responsible for FH binding to C3b (Figure 1D). Accordingly, SCR6-8 and SCR19-20, but not SCR1-4, bound VWF by surface plasmon resonance (SPR) (supplemental Figure 2), suggesting that VWF immobilization does not inactivate putative binding sites for FH. The identification of the FH domains 6 to 8 and 19 to 20 as the interacting partners does not exclude that other FH domains are implicated in complex formation.
Interaction of VWF and FH. (A) Colocalization of VWF and FH in WPBs of ECs. HUVEC were fixed with PFA (4%), permeabilized with Triton X-100 (0.5%), and stained for intracellular VWF and FH. For FH detection, a pool of 3 biotinylated monoclonal antibodies against FH or a biotinylated mouse IgG1 isotype control, followed by streptavidin-FITC, were used. For VWF, a rabbit polyclonal anti-human VWF IgG or a rabbit IgG control, followed by an Alexa Fluor 555–labeled goat anti-rabbit IgG, were used. Nuclei were stained with Hoechst 33342 dye (blue); a 63× oil-immersion objective was used for photography. (B) Cosecretion of FH and VWF by heme-stimulated HUVECs. Confluent HUVECs were incubated alone or in the presence of 100 µM heme in serum-free medium for 30 minutes at 37°C. VWF and FH were detected in culture supernatant by ELISA using commercially available purified proteins as standards. (C-D,F) Interaction between VWF and FH. VWF (10 nM; C) or anti-VWF antibody (D,F) were coated on 96-well microtiter plates. After blocking with Tris-buffered saline, 3% albumin, purified FH or recombinant FH fragments consisting of domains 1 to 4, 6 to 8, and 19 to 20 (C), or normal plasma (D,F) were incubated. In panel C, plasma was incubated in the presence of 300 mM NaCl to prevent formation of the VWF/FH complex during the incubation time (see supplemental Figure 4, available on the Blood Web site). FH and its fragments were detected with a polyclonal goat anti-FH antibody, a horseradish peroxidase–conjugated rabbit polyclonal anti-goat antibody and substrate. The polyclonal anti-FH IgG recognized equally FH and all recombinant FH fragments (supplemental Figure 1). (E) SPR analysis of FH binding to VWF. Association and dissociation of FH (1.5-3200 nM) with immobilized recombinant VWF (5500 RU) were followed in 10mM HEPES, 150 mM NaCl, Tween 0.005%, pH 7.4, for 360 seconds each, with a flow rate of 10 μL per minute. Curves depict RUs as a function of time. Data were fitted by the 1:1 Langmuir binding model with a drifting baseline. (E, inset) The equilibrium binding as the maximum signal measured at the end of the association phase as a function of the concentration of FH. Data were fitted with a 1-site nonlinear fit. Representative of 2 independent experiments. (F) (Inset) Protein G–coated sepharose beads where incubated with a polyclonal anti-VWF antibody, and then incubated with IgG-depleted normal plasma. FH was detected by western blotting under reduced conditions using an anti-FH antibody (lane 1, normal plasma; lane 2, type 3 VWD plasma; lane 3, FH-depleted serum). FITC, fluorescein isothiocyanate; HEPES, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; PFA, paraformaldehyde; RU, resonance unit.
Interaction of VWF and FH. (A) Colocalization of VWF and FH in WPBs of ECs. HUVEC were fixed with PFA (4%), permeabilized with Triton X-100 (0.5%), and stained for intracellular VWF and FH. For FH detection, a pool of 3 biotinylated monoclonal antibodies against FH or a biotinylated mouse IgG1 isotype control, followed by streptavidin-FITC, were used. For VWF, a rabbit polyclonal anti-human VWF IgG or a rabbit IgG control, followed by an Alexa Fluor 555–labeled goat anti-rabbit IgG, were used. Nuclei were stained with Hoechst 33342 dye (blue); a 63× oil-immersion objective was used for photography. (B) Cosecretion of FH and VWF by heme-stimulated HUVECs. Confluent HUVECs were incubated alone or in the presence of 100 µM heme in serum-free medium for 30 minutes at 37°C. VWF and FH were detected in culture supernatant by ELISA using commercially available purified proteins as standards. (C-D,F) Interaction between VWF and FH. VWF (10 nM; C) or anti-VWF antibody (D,F) were coated on 96-well microtiter plates. After blocking with Tris-buffered saline, 3% albumin, purified FH or recombinant FH fragments consisting of domains 1 to 4, 6 to 8, and 19 to 20 (C), or normal plasma (D,F) were incubated. In panel C, plasma was incubated in the presence of 300 mM NaCl to prevent formation of the VWF/FH complex during the incubation time (see supplemental Figure 4, available on the Blood Web site). FH and its fragments were detected with a polyclonal goat anti-FH antibody, a horseradish peroxidase–conjugated rabbit polyclonal anti-goat antibody and substrate. The polyclonal anti-FH IgG recognized equally FH and all recombinant FH fragments (supplemental Figure 1). (E) SPR analysis of FH binding to VWF. Association and dissociation of FH (1.5-3200 nM) with immobilized recombinant VWF (5500 RU) were followed in 10mM HEPES, 150 mM NaCl, Tween 0.005%, pH 7.4, for 360 seconds each, with a flow rate of 10 μL per minute. Curves depict RUs as a function of time. Data were fitted by the 1:1 Langmuir binding model with a drifting baseline. (E, inset) The equilibrium binding as the maximum signal measured at the end of the association phase as a function of the concentration of FH. Data were fitted with a 1-site nonlinear fit. Representative of 2 independent experiments. (F) (Inset) Protein G–coated sepharose beads where incubated with a polyclonal anti-VWF antibody, and then incubated with IgG-depleted normal plasma. FH was detected by western blotting under reduced conditions using an anti-FH antibody (lane 1, normal plasma; lane 2, type 3 VWD plasma; lane 3, FH-depleted serum). FITC, fluorescein isothiocyanate; HEPES, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; PFA, paraformaldehyde; RU, resonance unit.
We further studied FH-VWF binding in real-time using SPR. The injection of FH on a VWF-immobilized chip showed dose-dependent binding with an apparent KD of 180nM ± 20nM (Figure 1E). At physiological ionic strength, FH-VWF association and dissociation constants were 2.10 × 104 ± 0.39 × 104 (nM × s)−1 and 3.65 × 10−3 ± 0.35 × 10−3s−1, respectively. The FH-VWF interaction was pH and NaCl-dependent (supplemental Figures 3 and 4), suggesting that it is driven by electrostatic forces, as most of the interactions between complement proteins. Whether the interaction occurs in the WPB, at the time of secretion and/or on the surface of activated ECs and platelets remains to be investigated.
FH controls inappropriate complement activation by acting as a cofactor for FI in the cleavage and inactivation of C3b into iC3b, as shown by the dose-dependent appearance of the 64- and 43-kDa proteolysis bands of the α′ chain of C3b (Figure 2A). The presence of VWF was associated with enhanced FI-mediated inactivation of C3b at all FH concentrations tested, while the presence of HSA had no effect (Figure 2A). Accordingly, incubation of fixed low amounts of FH in the presence of increasing concentrations of VWF led to a dose-dependent increase in FH cofactor activity (Figure 2B). Incubation of VWF with C3b and FI, in the absence of FH, did not yield iC3b generation, indicating that VWF has no effect by itself on C3b degradation by FI but enhances FH cofactor activity.
Effect of the interaction between VWF and FH on FH cofactor activity, ADAMTS13-mediated VWF proteolysis, and VWF:RCo assay. (A-B) Cofactor activity of FH for CFI. FH (A, 0-32 nM; B, 13 nM), FI (17 nM), and C3b (8 nM) were incubated in the presence of VWF (A, 80 nM; B, 0-80 nM) or human serum albumin (HSA) (A, 80 nM) for 30 minutes at 37°C. The proteolysis of C3b into iC3b was analyzed by 10% SDS–polyacrylamide gel electrophoresis under reducing conditions using a goat anti-human C3 antibody. The presence of VWF enhanced FH cofactor activity resulting in increased cleavage of the C3b α′ chain into the α 65- and α 43-kDa fragments. (C) Effect of FH on ADAMTS13-mediated VWF proteolysis. Kinetics of digestion of VWF (10 nM) in the presence or absence of FH (645 nM) by recombinant ADAMTS13 (10 nM) in Tris-buffered saline. VWF digestion was carried out without shear stress and without denaturing agent. At selected times, the reaction was stopped by EDTA. The residual VWF was measured by a sandwich ELISA using a monoclonal antibody against the N-terminal site of VWF for capture and an anti–C-terminal antibody for detection. Plain curves represent the data fitted to the 1-phase exponential decay (r2 ≥ 0.87). Data depict means ± standard deviation of 3 independent experiments. (D) Distribution of VWF multimers. The samples recovered after 12 hours of incubation were separated by an SDS–2% agarose gel, transferred to a nitrocellulose membrane, and revealed using a polyclonal anti-VWF IgG coupled to horseradish peroxidase. The figure depicts the migration profile of VWF (10 nM) incubated alone, in the presence of ADAMTS13 (10 nM) with or without FH (645 nM). (E) Effect of FH on VWF:RCo assay. VWF (4 nM) incubated with purified FH (344 nM, 30 minutes at 37°C) and added on formol-fixed platelets in the presence of ristocetin. VWF:RCo was assessed using a commercial kit (Siemens) on an APAC4004 platelet aggregometer (Elitech). Percentage of VWF-mediated platelet aggregation was compared with normal human plasma. FI, complement factor I; RCo, ristocetin cofactor.
Effect of the interaction between VWF and FH on FH cofactor activity, ADAMTS13-mediated VWF proteolysis, and VWF:RCo assay. (A-B) Cofactor activity of FH for CFI. FH (A, 0-32 nM; B, 13 nM), FI (17 nM), and C3b (8 nM) were incubated in the presence of VWF (A, 80 nM; B, 0-80 nM) or human serum albumin (HSA) (A, 80 nM) for 30 minutes at 37°C. The proteolysis of C3b into iC3b was analyzed by 10% SDS–polyacrylamide gel electrophoresis under reducing conditions using a goat anti-human C3 antibody. The presence of VWF enhanced FH cofactor activity resulting in increased cleavage of the C3b α′ chain into the α 65- and α 43-kDa fragments. (C) Effect of FH on ADAMTS13-mediated VWF proteolysis. Kinetics of digestion of VWF (10 nM) in the presence or absence of FH (645 nM) by recombinant ADAMTS13 (10 nM) in Tris-buffered saline. VWF digestion was carried out without shear stress and without denaturing agent. At selected times, the reaction was stopped by EDTA. The residual VWF was measured by a sandwich ELISA using a monoclonal antibody against the N-terminal site of VWF for capture and an anti–C-terminal antibody for detection. Plain curves represent the data fitted to the 1-phase exponential decay (r2 ≥ 0.87). Data depict means ± standard deviation of 3 independent experiments. (D) Distribution of VWF multimers. The samples recovered after 12 hours of incubation were separated by an SDS–2% agarose gel, transferred to a nitrocellulose membrane, and revealed using a polyclonal anti-VWF IgG coupled to horseradish peroxidase. The figure depicts the migration profile of VWF (10 nM) incubated alone, in the presence of ADAMTS13 (10 nM) with or without FH (645 nM). (E) Effect of FH on VWF:RCo assay. VWF (4 nM) incubated with purified FH (344 nM, 30 minutes at 37°C) and added on formol-fixed platelets in the presence of ristocetin. VWF:RCo was assessed using a commercial kit (Siemens) on an APAC4004 platelet aggregometer (Elitech). Percentage of VWF-mediated platelet aggregation was compared with normal human plasma. FI, complement factor I; RCo, ristocetin cofactor.
Upon secretion by ECs, ultralarge VWF multimers are rapidly hydrolyzed by ADAMTS13.11,12 Addition of FH to an ADAMTS13-dependent VWF proteolysis assay resulted in a drastic reduction in VWF proteolysis (Figure 2C-D), in contrast to factor VIII (FVIII) which enhances VWF cleavage.13 The time to reach 50% hydrolysis of the initial VWF level was 10 hours ± 1 hour in the absence of FH vs 47 hours ± 2 hours in the presence of FH (Figure 2C). Accordingly, as depicted in Figure 2D upon VWF multimer analysis, the addition of FH was associated with increased amounts of high-molecular-weight multimers and reduced amounts of low-molecular-weight multimers. Moreover, addition of FH to VWF, at a physiological concentration ratio, was associated with a consistent 1.7-fold increase in VWF-mediated platelet aggregation (19.2% ± 6.7% vs 32.8% ± 8.4% in the absence or presence of FH, respectively, P = .044; Figure 2E). FH alone was devoid of an aggregating effect. Likewise, the presence of an excess of HSA (4 mg/mL) either with or without FH had no effect on VWF-mediated platelet aggregation (not shown). Of note, the presence of FH did not alter the capacity of VWF to bind collagen (supplemental Figure 5) or FVIII (not shown). Together, these results suggest that VWF-FH interaction facilitates VWF-mediated maintenance of hemostasis after injury.
In conclusion, we describe a novel type of interaction that implicates VWF and FH, which may participate in blood clotting at the site of injury by strengthening platelet aggregation and protects VWF from excessive proteolysis by ADAMTS13. In parallel, this interaction enhances the anti-inflammatory role of FH in regulating complement activation on activated or damaged endothelium. Our data provide a further association of VWF with the innate immune system.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Paulette Legendre for technical assistance (Institut National de la Santé et de la Recherche Médicale [INSERM] U770).
This work was supported by INSERM, Centre National de la Recherche Scientifique, and Université Pierre et Marie Curie Paris 6. Recombinant ADAMTS13 and recombinant VWF were gifts from Baxter Bioscience (Vienna, Austria) and recombinant FVIII (Helixate) from CSL-Behring (Marburg, Germany).
Authorship
Contribution: J.R., L.T.R., and S.L.-D. planned the work; J.R., L.T.R., J.D.D., Y.R., and M.I. performed experiments; L.H.-M., O.C., T.S.J., A.B.-D., and V.F.-B. contributed essential material; J.R., L.T.R., J.D.D., Y.R., S.V.K., V.F.-B., and S.L.-D. analyzed the data; and J.R., L.T.R., and S.L.-D. wrote the report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sébastien Lacroix-Desmazes, INSERM UMR S 872 Equipe 16, Centre de Recherche des Cordeliers, Paris, F-75006 France; e-mail: sebastien.lacroix-desmazes@crc.jussieu.fr; and Véronique Frémeaux-Bacchi, Service d’Immunologie Biologique, Hôpital Européen Georges Pompidou, 20-40 rue Leblanc, 75908 Paris cedex 15, France; e-mail: veronique.fremeaux-bacchi@egp.aphp.fr.
References
Author notes
J.R. and L.T.R. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal