Key Points
Rituximab may be a safe and effective therapy in patients with both large granular lymphocyte leukemia and rheumatoid arthritis.
LGLL in some patients with rheumatoid arthritis may be a reactive manifestation of chronic autoantigen stimulation rather than a true concomitant malignancy.
Abstract
T-cell large granular lymphocyte leukemia (LGLL) is a rare clonal disease often associated with rheumatoid arthritis (RA) and manifests chiefly as neutropenia and recurrent infections. Immunosuppressive agents are the mainstay of treatment, but long-term remissions are rare. We report 2 cases of LGLL in patients with RA successfully treated with rituximab, a monoclonal antibody specific of B cells and approved for treating RA. The first patient experienced a complete LGLL remission that was sustained during the 8-year follow-up after the first rituximab infusion. In the second patient, rituximab therapy was followed by immediate neutropenia recovery and then by marked shrinkage of the LGLL clone 1 year later. The paradoxical efficacy of this specific anti–B-cell drug on a monoclonal T-cell disease suggests that some cases of LGLL may be reactive manifestations of chronic autoantigen stimulation rather than true malignancies.
Introduction
T-cell large granular lymphocyte leukemia (LGLL) is a rare clonal disorder in which a monoclonal T-cell population accumulates in the blood, bone marrow, spleen, and liver.1 The main clinical manifestations are cytopenia (mostly neutropenia), splenomegaly, and recurrent infections.2 T-cell LGLL is associated with rheumatoid arthritis (RA) in up to one-third of cases.3
No large prospective trials have been conducted in LGLL.4 Low-dose methotrexate and short-term prednisone are the main first-line drugs.5,6 The overall 3-month response rate is only 55%, and most responders relapse after 1 year of treatment.7 Refractory disease may respond to cyclosporine, cyclophosphamide,4 or alemtuzumab.8,9 Complete remissions are rare.
Rituximab is a monoclonal anti-CD20 antibody approved for B-cell non-Hodgkin lymphoma10 and RA.11 Rituximab induces complete depletion of circulating B cells with no direct effects on T cells. No data have been published on rituximab therapy in patients with both RA and LGLL. Here, we describe 2 cases. This study is a retrospective review of clinical cases. No ethics committee consultation was required, but informed consent was obtained from both patients according to the Declaration of Helsinki.
Study design
Case 1
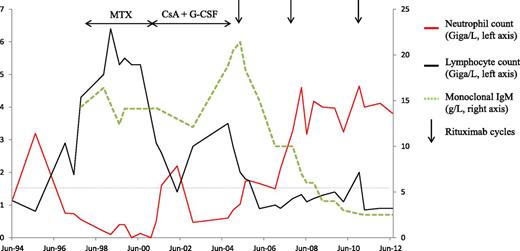
A woman born in 1932 was diagnosed in 1998 with erosive rheumatoid factor–positive RA. Subsequent tests were positive for anti-citrullinated protein antibody. Within a few months, severe neutropenia developed (Figure 1), and investigations showed a monotypic T-cell receptor (TCR)αβ+CD3+CD8+CD16+CD56−CD57+ T-cell LGL population in the blood and bone marrow. The absolute blood LGL count was 2.5 Giga (G)/L, representing 50% of total lymphocytes. A TCR-gene rearrangement study detected a monoclonal T-cell population. No splenomegaly or focal splenic abnormalities were seen by ultrasonography. A low-level serum IgMκ component was detected. No evidence of B-cell lymphoma was found by bone marrow smear, biopsy, and flow cytometry or by computed tomography. Methotrexate and low-dose prednisone were started and then changed in 2001 to cyclosporine 2 mg/kg/day combined with weekly granulocyte colony-stimulating factor (G-CSF) injections as the patient had profound agranulocytosis revealed by severe stomatitis. In 2004, chronic fever and weight loss developed. Extensive investigations found no evidence of infection, and the RA was quiescent. The cyclosporine was stopped, after which the neutropenia worsened, requiring daily G-CSF injections. The monoclonal IgM component level increased to 21 g/L, the κ/λ free light-chain ratio increased to 5, and massive κ Bence-Jones proteinuria appeared. Again, histological, cytological, and flow cytometry studies of bone marrow showed no signs of B-cell lymphoma. No lymphadenopathy was found by physical examination or computed tomography. Rituximab therapy was started in May 2005 (4 infusions of 375 mg/m2 each). The constitutional symptoms resolved rapidly, and the neutrophil blood count increased progressively until normalization after 4 months without further G-CSF injections. The monoclonal IgM component level decreased gradually. Two additional rituximab courses (each consisting of 2 1000-mg infusions) were given in 2008 and 2010 for RA flares. At the last evaluation in May 2013, the neutrophil count was normal at 3.2 G/L, the monoclonal IgMκ titer was 2.1 g/L, and the blood lymphocyte immunophenotype was normal. The hemoglobin level and platelet count were also normal. No monoclonal T-cell population was detectable by TCR-gene rearrangement study. Thus, the LGLL was in complete remission 8 years after the first rituximab infusion.
Patient 1 with RA. Neutropenia associated with LGLL and monoclonal IgM gammopathy were diagnosed in 1998. Low-dose methotrexate was given from 1998 to 2001 and then stopped because of agranulocytosis with severe stomatitis. Cyclosporine A and G-CSF were started in 2001 and then discontinued in 2004 because of chronic fever and weight loss attributed to monoclonal IgM progression. The first rituximab infusions were given in 2005 and ensured control of the joint symptoms, a decrease in monoclonal IgM titers, and prolonged complete remission of the leukemia. CsA, cyclosporine A; MTX, methotrexate.
Patient 1 with RA. Neutropenia associated with LGLL and monoclonal IgM gammopathy were diagnosed in 1998. Low-dose methotrexate was given from 1998 to 2001 and then stopped because of agranulocytosis with severe stomatitis. Cyclosporine A and G-CSF were started in 2001 and then discontinued in 2004 because of chronic fever and weight loss attributed to monoclonal IgM progression. The first rituximab infusions were given in 2005 and ensured control of the joint symptoms, a decrease in monoclonal IgM titers, and prolonged complete remission of the leukemia. CsA, cyclosporine A; MTX, methotrexate.
Case 2
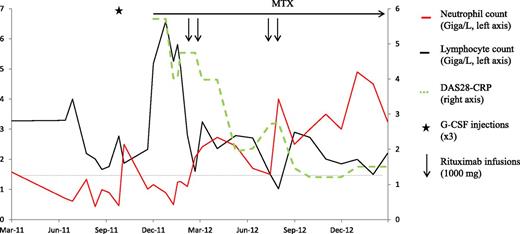
This man born in 1948 started experiencing inflammatory pain in the hands and feet in March 2011. Neutropenia was first detected in June 2011 (Figure 2). Hematological investigations disclosed a monotypic TCR-αβ+CD3+CD8+CD16+CD56−CD57+ LGL population representing 50% of blood and bone marrow lymphocytes (absolute blood LGL count, 2.1 G/L). TCR-Vβ flow cytometry showed a defective repertoire, and clonality was demonstrated by TCR-gene rearrangement analysis. He had no splenomegaly or lymphadenopathy. T-cell LGLL was diagnosed, but the only treatment was a G-CSF injection in September 2011. In November 2011, rheumatoid factor– and anti-citrullinated protein antibody–positive RA was diagnosed. Methotrexate and prednisone were started, but 3 months later the joint symptoms had worsened despite a prednisone dosage increase to 20 mg/d. The neutrophil blood count was 0.49 G/L, and the lymphocyte count was 6.6 G/L. A tumor necrosis factor α antagonist was not deemed appropriate given the clonal disease. Two 1000-mg rituximab infusions were given in February 2012 and rapidly induced a remission of the RA. The neutrophil count returned to normal after the first rituximab infusion and subsequently remained within the normal range. In July 2012, an increase in RA activity prompted a second rituximab course. The total lymphocyte count was normal (2.7 G/L), but the phenotype showed 40% monotypic CD3+CD8+CD57+ cells (absolute LGL count, 1.1 G/L). In January 2013, despite prednisone discontinuation since September 2012, the RA remained in remission. The neutrophil count was 3.6 G/L, and the CD3+CD8+CD57+ cells contributed only 13% of the total blood lymphocytes (LGL, 0.4 G/L). The monoclonal clone was still detectable by TCR-gene rearrangement molecular analysis.
Patient 2, a 63-year-old man diagnosed with T-cell LGLL in July 2011. Seropositive erosive RA was diagnosed in November 2011, and low-dose methotrexate and prednisone were started. Three months later, neither the joint disease nor the leukemia was improved. Two 1000-mg rituximab infusions provided a rapid remission of the joint disease accompanied with immediate neutrophil count normalization despite persistence of the monoclonal T-cell population. Six months later, a second rituximab course was given for exacerbation of the joint symptoms. In January 2013, the RA was still in remission despite prednisone discontinuation, neutrophil counts remained normal, and the leukemic cell count was substantially decreased. DAS28, RA disease activity score evaluating 28 joints; MTX, methotrexate.
Patient 2, a 63-year-old man diagnosed with T-cell LGLL in July 2011. Seropositive erosive RA was diagnosed in November 2011, and low-dose methotrexate and prednisone were started. Three months later, neither the joint disease nor the leukemia was improved. Two 1000-mg rituximab infusions provided a rapid remission of the joint disease accompanied with immediate neutrophil count normalization despite persistence of the monoclonal T-cell population. Six months later, a second rituximab course was given for exacerbation of the joint symptoms. In January 2013, the RA was still in remission despite prednisone discontinuation, neutrophil counts remained normal, and the leukemic cell count was substantially decreased. DAS28, RA disease activity score evaluating 28 joints; MTX, methotrexate.
Results and discussion
To our knowledge, our 2 patients are the first reported cases of successful rituximab therapy in RA-associated T-cell LGLL. The long-term follow-up in the first patient indicates that rituximab can produce a sustained improvement and even a complete remission of LGLL. Rituximab was well tolerated in both patients.
Two pathogenic theories have been proposed for T-cell LGLL. The initiating event leading to the monoclonal T-cell proliferation may be an acquired intrinsic molecular defect, such as a mutation. A recent study identified a somatic mutation in the signal transducer and activator of the transcription 3 gene in 31 (40%) of 77 LGLL patients, with a higher proportion (73%) in patients with RA.12 Such a mutation may cause the development of a monoclonal T-cell population.
Alternatively, the T-cell expansion may be driven by a combination of persistent antigenic stimulation and immunogenetic factors. LGLL cells exhibit the phenotype and genotype of cells subjected to chronic antigen activation, which may initiate clonal transformation. Serial measurements in 71 patients with T-cell LGLL showed that the dominant clonal population contracted or even disappeared over time in 26 (37%) patients, with or without a link to treatment, and was replaced by another clonal population expressing a different Vβ region.13 These findings suggest that, in some cases, development of the clonal population may be a reactive process unrelated to an intrinsic molecular defect. In RA, the effects of rituximab on the immune system may consist not only in transient B-cell depletion, but also in changes in the microenvironment and cytokine balance capable of impairing LGL clone survival without directly affecting the leukemic LGLs. This mechanism would explain the gradual shrinkage of the monoclonal population in our patients, until complete disappearance after several months in our first patient.
Late-onset neutropenia is a rare and usually transient complication of rituximab therapy in patients with malignant and rheumatic disorders.14,15 In some of these patients, an increase in CD3+CD8+CD57+ T-cell LGLs in the peripheral blood and bone marrow was described, with no monoclonality by TCR gene analysis.16,17
As illustrated by our first patient, T-cell LGLL is frequently associated with concomitant low-grade B-cell dyscrasias, such as monoclonal gammopathy of unknown significance or chronic lymphocytic leukemia, suggesting that a common antigen may drive both T- and B-cell proliferation.18
In conclusion, rituximab therapy may be safe and effective in LGLL-related neutropenia in RA patients and may produce a long-term complete remission of LGLL. The paradoxical efficacy of this specific anti–B-cell therapy on a T-cell monoclonal disease suggests that, in some cases, T-cell LGLL may be a reactive manifestation of chronic autoantigen stimulation rather than a true malignancy. Studies are warranted to assess our preliminary findings.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the local tumor tissue biobank “Tumorothèque de Brest” and the Brest Biological Resources Center (“CRB Santé de Brest”) for providing the frozen bone marrow and blood samples.
Authorship
Contribution: D.C., V.D.-P., S.J.-J., T.M., V.U., C.B., N.D.-G., and A.S. participated in the management of these two patients; D.C. and A.S. wrote the manuscript; and D.C., V.D.-P., S.J.-J., T.M., V.U., C.B., N.D.-G., and A.S. revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Divi Cornec, Service de Rhumatologie, Hôpital de la Cavale Blanche, BP 824, F 29609 Brest cedex, France; e-mail: divi.cornec@chu-brest.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal