In this issue of Blood, Lu et al shed light on the role of proviral integration sites of Moloney 2 (Pim2) kinase in multiple myeloma and delineate the mechanisms by which it mediates clonal plasma cells proliferation. The authors identify the tumor suppressor tuberous sclerosis 2TSC2 as the direct phosphorylation substrate of Pim2, suppressing its GTPase activity toward the small G-protein Rheb within the mammalian target of rapamycin C1 (mTORC1) complex. Based on this work, Pim2 inhibitors may indeed be the missing “torc” that pins down myeloma cells proliferation.1
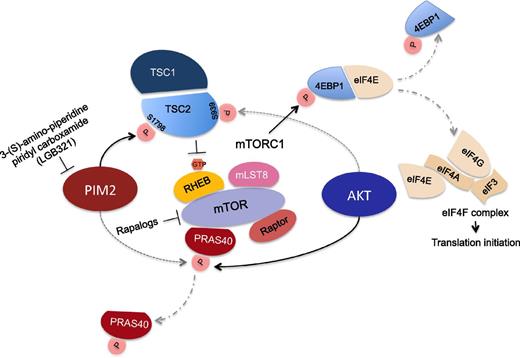
Pim2 and Akt kinase regulation of mTORC1: TSC2 (forms a complex with TSC1) inhibits the nutrient-mediated or growth factor-stimulated phosphorylation of S6K1 and 4EBP1 and the assembly of the elongation initiation factor 4E eIF4F complex comprising eIF4E, eIF4G, and eIF4A by negatively regulating mTORC1 signaling. TSC2 acts as a GTPase-activating protein (GAP) for the small GTPase RHEB, a direct activator of the protein kinase activity of mTORC1. See the article by Lu et al beginning on page 1610 where it is reported that Pim2 directly phosphorylates TSC2 on Ser-1798 (relieving its suppression on RHEB in mTORC1; solid line) and far less efficiently phosphorylates PRAS40 (dotted line), leading to its release from mTORC1 (PRAS40 suppresses mTORC1 activation). In contrast, PRAS40 is the main effector of Akt, which phosphorylates TSC2 on Ser-939 and Thr-1462.
Pim2 and Akt kinase regulation of mTORC1: TSC2 (forms a complex with TSC1) inhibits the nutrient-mediated or growth factor-stimulated phosphorylation of S6K1 and 4EBP1 and the assembly of the elongation initiation factor 4E eIF4F complex comprising eIF4E, eIF4G, and eIF4A by negatively regulating mTORC1 signaling. TSC2 acts as a GTPase-activating protein (GAP) for the small GTPase RHEB, a direct activator of the protein kinase activity of mTORC1. See the article by Lu et al beginning on page 1610 where it is reported that Pim2 directly phosphorylates TSC2 on Ser-1798 (relieving its suppression on RHEB in mTORC1; solid line) and far less efficiently phosphorylates PRAS40 (dotted line), leading to its release from mTORC1 (PRAS40 suppresses mTORC1 activation). In contrast, PRAS40 is the main effector of Akt, which phosphorylates TSC2 on Ser-939 and Thr-1462.
In 1984, H. Theo Cuypers described a recurrent retroviral integration site in murine leukemia virus (MuLV)-induced T-cell lymphomas that they named PIM1 for the proviral integration site in MuLV.2 Further studies in Eμ-Myc mice identified PIM1, along with PIM2 and PIM3, as oncogenes that promote MYC-induced lymphomagenesis.3 The Pim proteins are constitutively active serine/threonine kinases that are ubiquitously expressed with predominance for Pim1 in hematopoietic cells and for Pim2 in hematological malignancies including acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), diffuse large B-cell, and mantle cell lymphomas.4 The physiological activity of Pim kinases is mediated through the phosphorylation of large number of substrates, many of which are shared with Akt, and include transcriptional regulators (Myc, Myb), cell cycle regulators (p21, p27, CDC25A, and CDC25C), eukaryotic translation (4EBP1), and apoptosis mediators (Bad; also known as BCL2-associated agonist of cell death). The tumorigenic effects of Pim kinases are perpetuated through proproliferative mechanisms including potentiation of MYC transcriptional activity, enhancement of cap-dependent translation, and cell cycle progression, as well as antiapoptotic effects with up-regulation of MCL1 and Bad phosphorylation.
A potential role for Pim2 kinase in multiple myeloma (MM) was first reported by James Claudio in 2002, where 5′ end single-pass sequencing identified the Pim2 gene to be highly expressed in myeloma primary cells and cell lines.5 Later on, Asano et al6 reported that Pim2 protein was highly expressed in several myeloma cell lines and in plasma cells in bone marrow biopsies of myeloma patients. Importantly, they also described that Pim2 mRNA and protein were induced in cocultures of myeloma cells with bone marrow stromal cells or osteoclasts. This up-regulation of Pim2 is driven by interleukin 6 and tumor necrosis factor (TNF) family cytokines (TNF-α, B-cell activating factor [BAFF], and a proliferation-inducing ligand [APRIL]) through signal transducer and activator of transcription 3 and nuclear factor κB activation. The current study,1 confirmed the elevated Pim mRNA expression in hematological malignancies and in particular Pim2 in MM cells. Treatment with Pim kinase inhibitor (LGB321) or Pim2 short hairpin RNA silencing studies also supported the previous finding by Asano et al6 and others,7 implicating Pim2 in MM cell proliferation. Importantly, the authors dissected the mechanisms that mediate Pim2 proliferative effects and unearthed a novel mechanism of regulation of mTOR signaling in MM. Mechanistically, the authors identified TSC2 (negative regulator of mTOR-C1) as a direct substrate of Pim2 kinase with TSC2 phosphorylation on Ser-1798. This phosporylation relieves the small G-protein Rheb, within the mTORC1 complex, from TSC2 GTPase activity and promotes mTORC1 activation (see figure).
However, do we need another mTORC1 inhibitor in MM? Are PIM2 inhibitors merely another Akt-kinase “like” inhibitor or another class of rapalogs? Cognisant of the therapeutic challenges and shortcomings encountered thus far with this class of drugs (mTROC1 inhibitors),8 the studies by Lu et al provide further evidence why Pim2 inhibitors may complement or synergize with Akt inhibitors and/or possibly rapalogs. First, and unlike Akt kinase, the authors demonstrate that Pim2 inhibition suppresses mTORC1 signaling mainly through direct TSC2 phosphorylation, whereas AKT kinase largely promotes mTORC1 signaling through PRAS40 phosphorylation (leading to its dissociation from the mTORC1 complex). In addition, Pim2 and Akt phosphorylate TSC2 on distinct sites (Ser-1798 and Ser-939/Thr-1462, respectively), suggesting that Pim2 and Akt inhibitors may have synergistic or cooperative activating effect on TSC2 with suppression of mTORC1 signaling. Indeed, work from Craig Thompson’s laboratory9 previously demonstrated that Pim2 maintained cell size independently of the PI3K/AKT pathway. Furthermore, and consistent with an additive rather than epistatic effect on TSC2, the previous work by Asano et al6 reported that treatment with the Pim2 inhibitor and LY294002 (PI3K inhibitor) cooperatively suppressed MM cells viability.
Is there any advantage to Pim2 inhibitors over rapalogs? Unlike rapalogs, and somehow intriguing, Pim2 inhibitors do not appear to increase Akt Ser-473 phosphorylation (feedback activation) despite similar suppression of mTORC1. The authors speculate that this difference may stem from an inhibitory effect of TSC1-TSC2 complex on mTORC2 (as well as mTORC1). This differential effect on Akt activation may represent a major advantage of Pim2 inhibitors over rapalogs. In addition, it is worth noting that Pim2 inhibitors, unlike rapalogs, are shown to equally suppress the proliferation of MM cell lines irrespective of Deptor expression. Furthermore, in view of the high Pim2 expression in MM cells (therapeutic window) and the fact that Pim2 knockout mice have a null phenotype, it is plausible to speculate that the toxicity profile of Pim2 inhibitors is likely far more tolerable, with lesser immunosuppressive effects, compared with that of rapalogs. This hypothesis will need to await the confirmatory rigorous testing of future clinical trials with Pim kinase inhibitors.
In summary, although mTORC1 activation was long recognized as a key driver of MM cell proliferation, the therapeutic intervention to pin down this giant have largely fallen short of expectations. The identification of Pim2 kinase as a direct regulator of the TSC2-mTORC1 and the promising prospects of the combination of Pim2 kinase and PI3K/AKT inhibitors may finally arm us with the sling that brings down Goliath!
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal