Key Points
Obinutuzumab is a novel type II anti-CD20 monoclonal antibody under early-stage clinical investigation.
Obinutuzumab plus CHOP or FC has an acceptable safety profile and is effective in relapsed/refractory follicular lymphoma.
Abstract
The safety and activity of obinutuzumab (GA101) plus chemotherapy in relapsed/refractory follicular lymphoma was explored in 56 patients. Participants received obinutuzumab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (G-CHOP; every 3 weeks for 6 to 8 cycles) or obinutuzumab plus fludarabine and cyclophosphamide (G-FC; every 4 weeks for 4 to 6 cycles). Patients were randomly assigned to either obinutuzumab 1600 mg on days 1 and 8 of cycle 1 followed by 800 mg on day 1 of subsequent cycles or 400 mg for all doses. Treatment responders were eligible for obinutuzumab maintenance every 3 months for up to 2 years. Grade 1/2 infusion-related reactions (IRRs) were the most common treatment-related adverse event (AE) (all grades: G-CHOP, 68%; G-FC, 82%). Grade 3/4 IRRs were rare (7%) and restricted to the first infusion. All patients received the planned obinutuzumab dose. Neutropenia was the most common treatment-related hematologic AE for G-CHOP (43%) and G-FC (50%). At induction end, 96% (27/28) of patients receiving G-CHOP (complete response [CR], 39% [11/28]) and 93% (26/28) receiving G-FC (CR, 50% [14 of 28]) achieved responses. G-CHOP and G-FC had an acceptable safety profile with no new or unexpected AEs, but G-FC was associated with more AEs than G-CHOP. Obinutuzumab plus chemotherapy resulted in 93% to 96% response rates, supporting phase 3 investigation. This trial was registered at www.clinicaltrials.gov as #NCT00825149.
Introduction
Rituximab-based immunochemotherapy is the backbone of treatment of non-Hodgkin lymphoma (NHL).1 Compared with chemotherapy, immunochemotherapy provides significant, sustained clinical benefits, including prolonged survival in patients with aggressive NHL,2-4 indolent NHL (iNHL),5-11 and chronic lymphocytic leukemia.12,13 Many patients who initially benefit from rituximab-based immunochemotherapy relapse, and some fail to respond. There is, therefore, a need to develop therapies with improved antitumor activity and better risk/benefit profiles for optimal treatment of B-cell malignancies.
Obinutuzumab (GA101) is a type II CD20-targeted monoclonal antibody (mAb) with enhanced direct cell death induction but reduced complement-dependent cytotoxicity.14 Obinutuzumab has been glycoengineered to decrease the fucose content of the Fc region, which increases its affinity for FcγRIIIa on effector cells, even low-affinity receptor variants. Consequently, antibody-dependent cell-mediated cytotoxicity is increased.14,15 In vitro studies have demonstrated different mechanisms of action for rituximab (type I nonglycoengineered mAb) and obinutuzumab (type II glycoengineered mAb), although both target the same antigen and have overlapping epitopes.14 Molecular differences between type I and type II mAbs and how they bind to their epitopes may lead to different functional properties; preclinical studies have demonstrated the superiority of obinutuzumab over rituximab and ofatumumab (type I mAbs) in killing lymphoma cells,14 with increases in direct cell death via caspase-independent, nonapoptotic, lysosomal mechanisms.16 The increased antitumor activity of obinutuzumab in human xenograft models has also been shown.14,17
Phase 1 and phase 2 studies in patients with NHL have demonstrated promising activity from single-agent obinutuzumab.18-21 Here we report the results of the phase 1b GAUDI study (BO21000), which explored the safety and activity of 2 doses of obinutuzumab combined with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or fludarabine and cyclophosphamide (FC) as induction therapy in patients with relapsed/refractory follicular lymphoma (FL).
Patients and methods
Study design
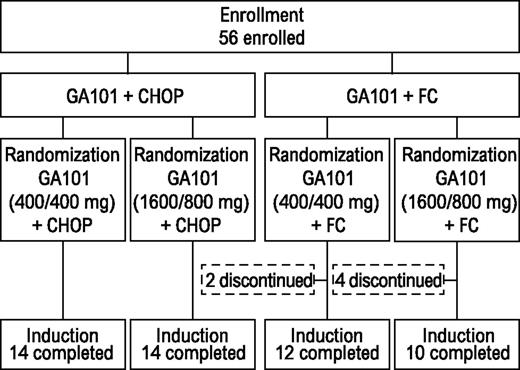
GAUDI was an open-label, multicenter, phase 1b study composed of patients with previously untreated or relapsed/refractory FL. Data only from the relapsed/refractory cohort are reported here. Patients with relapsed/refractory FL requiring treatment (as determined by the treating physician) received induction with either obinutuzumab plus CHOP (G-CHOP) every 3 weeks for 6 to 8 cycles or obinutuzumab plus FC (G-FC) every 4 weeks for 4 to 6 cycles. Chemotherapy choice (either CHOP or FC) was at investigator discretion and was based on the patient’s treatment history; the protocol required 28 patients to be treated with each chemotherapy regimen. Following assignment to G-CHOP or G-FC, patients were randomly assigned to either obinutuzumab 1600/800 mg (1600 mg on days 1 and 8 of cycle 1, 800 mg on day 1 of subsequent cycles) or obinutuzumab 400/400 mg (400 mg on days 1 and 8 of cycle 1, 400 mg on day 1 of subsequent cycles). These doses were based on previous pharmacokinetic analyses.18 Patients achieving complete responses (CRs) or partial responses (PRs) after induction were eligible for obinutuzumab maintenance therapy administered at the induction dose (every 3 months for up to 2 years or until progression).
The primary end point was safety. Secondary end points included overall response (OR) rate, CR rate, pharmacokinetics, peripheral B-cell depletion and recovery, and effects of Fcγ receptor polymorphisms on outcomes.
Patients
Eligible patients were age >18 years with CD20+ relapsed/refractory FL after receiving ≤2 prior chemotherapy/immunochemotherapy regimens. Refractory disease was defined as progressive or stable disease, or a PR or better response with progression for <6 months of any prior therapy. Patients had to have ≥1 bidimensionally measurable lesions (>1.5 cm), life expectancy >12 weeks, Eastern Cooperative Oncology Group performance status of 0-2, and a CD20+ biopsy (confirmed by central review). Biopsy of lymph nodes within 5 months prior to treatment was required to rule out transformation.
Exclusion criteria included prior anthracycline therapy (G-CHOP cohort), fludarabine or purine analog exposure within 1 year (G-FC cohort), prior rituximab use within 56 days, prior radioimmunotherapy within 3 months, prior investigational mAb use within 6 months, participation in a clinical study 30 days prior to study entry, prior allogeneic bone marrow transplant, and active bacterial or viral infections or positive serologic tests for hepatitis B, hepatitis C, or HIV. Patients had to have adequate renal function (creatinine clearance >50 mL/min for G-CHOP, >60 mL/min for G-FC), liver function (alanine aminotransferase and aspartate aminotransferase ≤2.5× upper limit of normal), and appropriate hematologic parameters (platelets ≥100 × 109/L; neutrophils ≥1.5 × 109/L; hemoglobin ≥10 g/dL, unless due to lymphoma or prior therapy).
All patients provided written informed consent. This investigation was conducted according to Declaration of Helsinki and Good Clinical Practice principles. The protocol was approved by institutional ethics committees. This trial is registered at Clinicaltrials.gov (NCT00825149).
Treatment
Obinutuzumab was infused on days 1 and 8 of cycle 1 and day 1 of subsequent cycles as previously described.18 Patients were premedicated with acetaminophen and an antihistamine before obinutuzumab infusion. The first 3 patients in each cohort were restricted to obinutuzumab 400/400 mg and monitored until day 15 for significant unexpected adverse events (AEs). When none were observed, randomization commenced. In the G-CHOP cohort, patients received their first dose of the 5-day course of prednisone (100 mg) prior to initiating obinutuzumab infusion. In the G-FC cohort, patients with previous severe infusion-related reactions (IRRs) to rituximab or on first obinutuzumab infusion received corticosteroid premedication at the investigator’s discretion.
CHOP (doxorubicin, 50 mg/m2 intravenously on day 1; vincristine, 1.4 mg/m2 capped at 2 mg intravenously on day 1; cyclophosphamide, 750 mg/m2 intravenously on day 1; prednisone, 100 mg per day orally on days 1 to 5) and FC (fludarabine, 25 mg/m2 per day intravenously on days 1 to 3; cyclophosphamide, 250 mg/m2 intravenously on days 1 to 3) were administered immediately after obinutuzumab. For the G-CHOP cohort, use of antimicrobial prophylaxis and granulocyte colony-stimulating factor (G-CSF) was at the investigator’s discretion. The G-FC cohort received mandatory Pneumocystis carinii prophylaxis and antiviral prophylaxis against herpes simplex and varicella zoster reactivation. The total number of induction cycles delivered (6 to 8 in the G-CHOP arm; 4 to 6 in the G-FC arm) was according to standard institutional practice.
Assessments
Clinical examination—assessments of blood count, serum chemistry, human antihuman antibodies, and pharmacokinetics—and peripheral blood immunophenotyping were performed on days 1 and 8 of cycle 1 and day 1 of subsequent cycles. Human antichimeric antibodies were evaluated on day 1 of cycle 1. AEs were graded per National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. Clinical responses were assessed after cycle 3 in the G-FC cohort, after cycle 4 in the G-CHOP cohort, and at treatment end. Responses were assessed on the basis of computed tomography scans. Positron emission tomography scans were exploratory. Unconfirmed CRs were considered PRs per revised response criteria for malignant lymphoma.22
Statistical analysis
After physicians assigned patients to G-CHOP or G-FC, patients were randomly assigned to obinutuzumab 400/400 mg or 1600/800 mg. All patients who received ≥1 obinutuzumab doses were analyzed for safety and efficacy. Baseline and demographic characteristics are summarized descriptively. Patients with CR or PR were regarded as responders. Response rates and 95% Clopper-Pearson confidence intervals (CIs) were estimated for each obinutuzumab dose group and chemotherapy regimen. Statistical analyses were conducted using SAS (SAS Institute Inc., Cary, NC) or S-PLUS (Tibco Software, Inc., Palo Alto, CA).
Results
Patient characteristics
Fifty-six patients with relapsed/refractory FL received obinutuzumab plus CHOP (n = 28) or FC (n = 28) (Table 1). Within each immunochemotherapy cohort, the 1600/800-mg and 400/400-mg arms were well balanced for disease prognostic factors, including time from diagnosis, intermediate and high Follicular Lymphoma International Prognostic Index (FLIPI) score, and number of prior treatment lines. Compared with the G-CHOP cohort, the G-FC cohort had received a greater number of prior antilymphoma regimens. Although a maximum of 2 previous chemotherapy/immunochemotherapy regimens was permitted, patients may have received additional nonchemotherapy regimens, such as antibody monotherapy. At study entry, a higher proportion of patients in the G-FC cohort had advanced disease, rituximab-refractory disease, and high FLIPI score compared with the G-CHOP cohort.
Baseline patient and disease characteristics
| Characteristic . | G-CHOP 400/400 mg (n = 14) . | G-CHOP 1600/800 mg (n = 14) . | G-CHOP total (n = 28) . | G-FC 400/400 mg (n = 14) . | G-FC 1600/800 mg (n = 14) . | G-FC total (n = 28) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Age, y | ||||||||||||
| Median | 61.0 | 66.0 | 62.5 | 61.5 | 60.5 | 61.0 | ||||||
| Range | 32-75 | 34-75 | 32-75 | 48-77 | 45-77 | 45-77 | ||||||
| Male | 5 | 36 | 6 | 43 | 11 | 39 | 7 | 50 | 10 | 71 | 17 | 61 |
| Ann Arbor stage at study entry | ||||||||||||
| I-II | 3 | 21 | 6 | 43 | 9 | 32 | 4 | 29 | 1 | 7 | 5 | 18 |
| III-IV | 11 | 79 | 8 | 57 | 19 | 68 | 10 | 71 | 13 | 93 | 23 | 82 |
| Bulky disease (≥7 cm) | 7 | 50 | 2 | 14 | 9 | 32 | 1 | 7 | 4 | 29 | 5 | 18 |
| No. of patients with bone marrow involvement of total No. of patients in subgroup | 5/14 | 36 | 2/14 | 14 | 7/28 | 25 | 2/13 | 15 | 5/14 | 36 | 7/27 | 26 |
| FLIPI score at study entry | ||||||||||||
| Low (0-1) | 5 | 36 | 3 | 21 | 8 | 29 | 5 | 36 | 3 | 21 | 8 | 29 |
| Intermediate (2) | 5 | 36 | 9 | 64 | 14 | 50 | 4 | 29 | 5 | 36 | 9 | 32 |
| High (3-5) | 4 | 29 | 2 | 14 | 6 | 21 | 5 | 36 | 6 | 43 | 11 | 39 |
| Time from first diagnosis, months | ||||||||||||
| Median | 38 | 34 | 36 | 51 | 49 | 51 | ||||||
| Range | 19-229 | 7-88 | 7-229 | 14-181 | 19-305 | 14-305 | ||||||
| No. of prior treatment lines | ||||||||||||
| Median | 1 | 1 | 1 | 2 | 2 | 2 | ||||||
| Range | 1-3 | 1-2 | 1-3 | 1-4 | 1-6 | 1-6 | ||||||
| Refractory to last treatment | 1 | 7 | 4 | 29 | 5 | 18 | 2 | 14 | 5 | 36 | 7 | 25 |
| No. of prior rituximab treatment lines | ||||||||||||
| Median | 1 | 1 | 1 | 1 | 1.5 | 1 | ||||||
| Range | 1-2 | 1-2 | 1-2 | 1-2 | 0-3 | 0-3 | ||||||
| Rituximab refractory | 1 | 7 | 3 | 21 | 4 | 14 | 4 | 29 | 6 | 43 | 10 | 36 |
| Characteristic . | G-CHOP 400/400 mg (n = 14) . | G-CHOP 1600/800 mg (n = 14) . | G-CHOP total (n = 28) . | G-FC 400/400 mg (n = 14) . | G-FC 1600/800 mg (n = 14) . | G-FC total (n = 28) . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Age, y | ||||||||||||
| Median | 61.0 | 66.0 | 62.5 | 61.5 | 60.5 | 61.0 | ||||||
| Range | 32-75 | 34-75 | 32-75 | 48-77 | 45-77 | 45-77 | ||||||
| Male | 5 | 36 | 6 | 43 | 11 | 39 | 7 | 50 | 10 | 71 | 17 | 61 |
| Ann Arbor stage at study entry | ||||||||||||
| I-II | 3 | 21 | 6 | 43 | 9 | 32 | 4 | 29 | 1 | 7 | 5 | 18 |
| III-IV | 11 | 79 | 8 | 57 | 19 | 68 | 10 | 71 | 13 | 93 | 23 | 82 |
| Bulky disease (≥7 cm) | 7 | 50 | 2 | 14 | 9 | 32 | 1 | 7 | 4 | 29 | 5 | 18 |
| No. of patients with bone marrow involvement of total No. of patients in subgroup | 5/14 | 36 | 2/14 | 14 | 7/28 | 25 | 2/13 | 15 | 5/14 | 36 | 7/27 | 26 |
| FLIPI score at study entry | ||||||||||||
| Low (0-1) | 5 | 36 | 3 | 21 | 8 | 29 | 5 | 36 | 3 | 21 | 8 | 29 |
| Intermediate (2) | 5 | 36 | 9 | 64 | 14 | 50 | 4 | 29 | 5 | 36 | 9 | 32 |
| High (3-5) | 4 | 29 | 2 | 14 | 6 | 21 | 5 | 36 | 6 | 43 | 11 | 39 |
| Time from first diagnosis, months | ||||||||||||
| Median | 38 | 34 | 36 | 51 | 49 | 51 | ||||||
| Range | 19-229 | 7-88 | 7-229 | 14-181 | 19-305 | 14-305 | ||||||
| No. of prior treatment lines | ||||||||||||
| Median | 1 | 1 | 1 | 2 | 2 | 2 | ||||||
| Range | 1-3 | 1-2 | 1-3 | 1-4 | 1-6 | 1-6 | ||||||
| Refractory to last treatment | 1 | 7 | 4 | 29 | 5 | 18 | 2 | 14 | 5 | 36 | 7 | 25 |
| No. of prior rituximab treatment lines | ||||||||||||
| Median | 1 | 1 | 1 | 1 | 1.5 | 1 | ||||||
| Range | 1-2 | 1-2 | 1-2 | 1-2 | 0-3 | 0-3 | ||||||
| Rituximab refractory | 1 | 7 | 3 | 21 | 4 | 14 | 4 | 29 | 6 | 43 | 10 | 36 |
FLIPI, Follicular Lymphoma International Prognostic Index.
Patient disposition and treatment exposure
All patients receiving G-CHOP completed induction (Figure 1). Two patients (14%) from the G-FC 400/400-mg arm discontinued therapy because of AEs. Four (29%) from the G-FC 1600/800-mg arm discontinued because of AEs (n = 3) or insufficient response (n = 1). In both G-CHOP arms, all patients received ≥6 treatment cycles. In the G-FC arms, most patients received the minimum of 4 cycles of therapy. Two patients in each dose group withdrew during cycles 1 to 3. Two additional patients withdrew from the high-dose group during cycles 4 to 6.
Safety
In the G-CHOP cohort, the most frequent treatment-related hematologic AE was neutropenia (29% in the 400/400-mg arm; 57% in the 1600/800-mg arm; Table 2). Grade 3/4 neutropenia was documented in 29% and 50% of patients in the 400/400-mg and 1600/800-mg arms, respectively, lasting 9 days on average. One patient (7%) in the G-CHOP 1600/800-mg dose group experienced grade 3/4 febrile neutropenia. Most patients with grade 3/4 neutropenia (7/11) received G-CSF support; other grade 3/4 hematologic AEs were not experienced by >1 patient. The most common treatment-related nonhematologic AE was IRR (64% in both G-CHOP arms; Table 3). The 1600/800-mg G-CHOP arm had a higher infection incidence than the 400/400-mg arm (57% vs 29%), but grade 3/4 infection incidences were identical for both doses (21%; 3 patients each). Eight patients (4 in each arm) required dose reduction of ≥1 CHOP component because of AEs (5 for neuropathy; 1 each for neutropenia, infection, allergic rhinitis). Most dose reductions were of vincristine (5 patients). Eleven (6%) of 190 cycles delivered were delayed in 8 patients for neutropenia/infection. All G-CHOP–treated patients completed treatment.
Hematologic AEs
| Patients . | Treatment-related (all grades) . | Grade 3/4 (related or unrelated) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| G-CHOP | ||||||||||||
| Neutropenia | 4 | 29 | 8 | 57 | 12 | 43 | 4 | 29 | 7 | 50 | 11 | 39 |
| Febrile neutropenia | — | 1 | 7 | 1 | 4 | — | 1 | 7 | 1 | 4 | ||
| Thrombocytopenia | — | 1 | 7 | 1 | 4 | — | 1 | 7 | 1 | 4 | ||
| Anemia | 3 | 21 | 3 | 21 | 6 | 21 | 1 | 7 | — | 1 | 4 | |
| Leukopenia | — | 1 | 7 | 1 | 4 | — | 1 | 7 | 1 | 4 | ||
| G-FC | ||||||||||||
| Neutropenia | 9 | 64 | 5 | 36 | 14 | 50 | 9 | 64 | 5 | 36 | 14 | 50 |
| Febrile neutropenia | — | 1 | 7 | 1 | 4 | — | 2 | 14 | 2 | 7 | ||
| Thrombocytopenia | 2 | 14 | 4 | 29 | 6 | 21 | 3 | 21 | 4 | 29 | 7 | 25 |
| Anemia | 1 | 7 | — | 1 | 4 | 2 | 14 | — | 2 | 7 | ||
| Leukopenia | — | 1 | 7 | 1 | 4 | — | 1 | 7 | 1 | 4 | ||
| Patients . | Treatment-related (all grades) . | Grade 3/4 (related or unrelated) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| G-CHOP | ||||||||||||
| Neutropenia | 4 | 29 | 8 | 57 | 12 | 43 | 4 | 29 | 7 | 50 | 11 | 39 |
| Febrile neutropenia | — | 1 | 7 | 1 | 4 | — | 1 | 7 | 1 | 4 | ||
| Thrombocytopenia | — | 1 | 7 | 1 | 4 | — | 1 | 7 | 1 | 4 | ||
| Anemia | 3 | 21 | 3 | 21 | 6 | 21 | 1 | 7 | — | 1 | 4 | |
| Leukopenia | — | 1 | 7 | 1 | 4 | — | 1 | 7 | 1 | 4 | ||
| G-FC | ||||||||||||
| Neutropenia | 9 | 64 | 5 | 36 | 14 | 50 | 9 | 64 | 5 | 36 | 14 | 50 |
| Febrile neutropenia | — | 1 | 7 | 1 | 4 | — | 2 | 14 | 2 | 7 | ||
| Thrombocytopenia | 2 | 14 | 4 | 29 | 6 | 21 | 3 | 21 | 4 | 29 | 7 | 25 |
| Anemia | 1 | 7 | — | 1 | 4 | 2 | 14 | — | 2 | 7 | ||
| Leukopenia | — | 1 | 7 | 1 | 4 | — | 1 | 7 | 1 | 4 | ||
Related nonhematologic AEs (all grades occurring in >2 [15%] of patients in either cohort)
| Patients . | G-CHOP . | G-FC . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| IRR | 9 | 64 | 9 | 64 | 18 | 64 | 10 | 71 | 12 | 86 | 22 | 79 |
| Infections and infestations | 4 | 29 | 8 | 57 | 12 | 43 | 3 | 21 | 6 | 43 | 9 | 32 |
| Constipation | 4 | 29 | 2 | 14 | 6 | 21 | 3 | 21 | 2 | 14 | 5 | 18 |
| Nausea | 6 | 43 | 4 | 29 | 10 | 36 | 5 | 36 | 6 | 43 | 11 | 39 |
| Fatigue | 2 | 14 | 3 | 21 | 5 | 18 | 1 | 7 | 2 | 14 | 3 | 11 |
| Diarrhea | 6 | 43 | 3 | 21 | 9 | 32 | 2 | 14 | 0 | 0 | 2 | 7 |
| Vomiting | 3 | 21 | 1 | 7 | 4 | 14 | 1 | 7 | 1 | 7 | 2 | 7 |
| Dyspepsia | 3 | 21 | 2 | 14 | 5 | 18 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alopecia | 4 | 29 | 3 | 21 | 7 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucosal inflammation | 1 | 7 | 3 | 21 | 4 | 14 | 1 | 7 | 1 | 7 | 2 | 7 |
| Headache | 1 | 7 | 1 | 7 | 2 | 7 | 2 | 14 | 3 | 21 | 5 | 18 |
| Pyrexia | 2 | 14 | 1 | 7 | 3 | 11 | 4 | 29 | 1 | 7 | 5 | 18 |
| Asthenia | 0 | 0 | 3 | 21 | 3 | 11 | 3 | 21 | 3 | 21 | 6 | 21 |
| Patients . | G-CHOP . | G-FC . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| IRR | 9 | 64 | 9 | 64 | 18 | 64 | 10 | 71 | 12 | 86 | 22 | 79 |
| Infections and infestations | 4 | 29 | 8 | 57 | 12 | 43 | 3 | 21 | 6 | 43 | 9 | 32 |
| Constipation | 4 | 29 | 2 | 14 | 6 | 21 | 3 | 21 | 2 | 14 | 5 | 18 |
| Nausea | 6 | 43 | 4 | 29 | 10 | 36 | 5 | 36 | 6 | 43 | 11 | 39 |
| Fatigue | 2 | 14 | 3 | 21 | 5 | 18 | 1 | 7 | 2 | 14 | 3 | 11 |
| Diarrhea | 6 | 43 | 3 | 21 | 9 | 32 | 2 | 14 | 0 | 0 | 2 | 7 |
| Vomiting | 3 | 21 | 1 | 7 | 4 | 14 | 1 | 7 | 1 | 7 | 2 | 7 |
| Dyspepsia | 3 | 21 | 2 | 14 | 5 | 18 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alopecia | 4 | 29 | 3 | 21 | 7 | 25 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucosal inflammation | 1 | 7 | 3 | 21 | 4 | 14 | 1 | 7 | 1 | 7 | 2 | 7 |
| Headache | 1 | 7 | 1 | 7 | 2 | 7 | 2 | 14 | 3 | 21 | 5 | 18 |
| Pyrexia | 2 | 14 | 1 | 7 | 3 | 11 | 4 | 29 | 1 | 7 | 5 | 18 |
| Asthenia | 0 | 0 | 3 | 21 | 3 | 11 | 3 | 21 | 3 | 21 | 6 | 21 |
In the G-FC cohort, the most frequent hematologic AE was neutropenia (64% in the 400/400-mg arm; 36% in the 1600/800-mg arm; all were grade 3/4). Two patients (14%) in the 1600/800-mg arm experienced febrile neutropenia (Table 2) lasting 7 and 10 days. Twelve (86%) of 14 patients with grade 3/4 neutropenia received G-CSF support. Seven patients (25%) had grade 3/4 thrombocytopenia, with a median duration of 15 days; 1 patient required treatment. Grade 3/4 anemia occurred in 2 patients in the 400/400-mg arm. The most frequent treatment-related nonhematologic AE was IRR occurring in 71% in the 400/400-mg arm and 86% in the 1600/800-mg arm (Table 3). Infection incidences were higher in the 1600/800-mg than the 400/400-mg arm (all grades, 43% vs 21%; grade 3/4, 29% vs 7%, respectively). Five patients in each G-FC arm required cyclophosphamide and fludarabine dose reduction for AE management. Fourteen (10%) of 135 cycles delivered were delayed in 10 patients for hematologic AEs or infections. Five patients discontinued G-FC for AEs: 2 in the 400/400-mg arm for grade 4 neutropenia and 3 in the 1600/800-mg arm (1 each for severe neutropenia, mild rash, severe infection).
G-CHOP (68%) and G-FC cohorts (71%) had similar incidences of grade 3/4 AEs, with no relevant differences between arms in each cohort. Grade 3/4 nonhematologic AEs occurring in >1 patient per chemotherapy cohort are shown in Table 4.
Grade 3/4 nonhematologic AEs (occurring in >1 patient per chemotherapy cohort)
| Patients . | G-CHOP . | G-FC . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Infections | 3 | 21 | 3 | 21 | 6 | 21 | 1 | 7 | 4 | 29 | 5 | 18 |
| IRR | 1 | 7 | 1 | 7 | 2 | 7 | 1 | 7 | 1 | 7 | 2 | 7 |
| Vomiting | 1 | 7 | 1 | 7 | 2 | 7 | — | — | — | |||
| Patients . | G-CHOP . | G-FC . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| Infections | 3 | 21 | 3 | 21 | 6 | 21 | 1 | 7 | 4 | 29 | 5 | 18 |
| IRR | 1 | 7 | 1 | 7 | 2 | 7 | 1 | 7 | 1 | 7 | 2 | 7 |
| Vomiting | 1 | 7 | 1 | 7 | 2 | 7 | — | — | — | |||
Serious AEs (SAEs) were reported in 29% and 25% of patients in the G-CHOP and G-FC cohorts, respectively. There were no relevant differences in specific infections between arms in each cohort. No treatment-related deaths were reported.
IRRs occurred mainly during first infusion (8 patients in each dose arm for G-CHOP; 10 patients in each dose arm for G-FC). No more than 3 patients in either cohort experienced IRRs at any subsequent infusion. One patient in each cohort experienced a grade 3/4 IRR. All patients received the planned full dose of obinutuzumab.
Three patients were positive for anti-rituximab human antichimeric antibodies at screening, which was confirmed on second testing (2 patients, G-CHOP; 1 patient, G-FC); all responded to treatment. At treatment end, no patient had detectable human antihuman antibodies, including the 1 patient who had tested positive at screening.
Efficacy
For G-CHOP, the end-of-induction OR rate was 93% (95% confidence interval [CI], 66.1% to 99.8%) in the 400/400-mg arm (14% CR; 95% CI, 1.8% to 42.8%) and 100% (95% CI, 76.8% to 100.0%) in the 1600/800-mg arm (64% CR; 95% CI, 35.1% to 87.2%) (Table 5). For G-FC, the OR rate was 100% (95% CI, 76.8% to 100.0%) in the 400/400-mg arm (79% CR; 95% CI, 49.2% to 95.3%) and 86% (95% CI, 57.2% to 98.2%) in the 1600/800-mg arm (21% CR; 95% CI, 4.7% to 50.8%). In the G-CHOP cohort, 25% (1/4) of rituximab-refractory patients achieved CR. In the G-FC cohort, 30% (3/10) achieved CR. All rituximab-refractory patients achieved at least PR. Patients expressing unfavorable FcγRIIIa receptor variants (phenylalanine/phenylalanine and valine/phenylalanine) had CR rates of 33% (7/21) (G-CHOP) and 55% (11/20) (G-FC). The CR rates for patients with the valine/valine receptor variant in the G-CHOP and G-FC cohorts were 75% (3/4) and 67% (4/6), respectively.
End of induction therapy response rates
| Patients . | G-CHOP . | G-FC . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| ORR | 13 | 93 | 14 | 100 | 27 | 96 | 14 | 100 | 12 | 86 | 26 | 93 |
| CR | 2 | 14 | 9 | 64 | 11 | 39 | 11 | 79 | 3 | 21 | 14 | 50 |
| PR | 11 | 79 | 5 | 36 | 16 | 57 | 3 | 21 | 9 | 64 | 12 | 43 |
| SD | 1 | 7 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| PD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7 | 1 | 4 | |
| Patients . | G-CHOP . | G-FC . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | 400/400 mg (n = 14) . | 1600/800 mg (n = 14) . | Total (n = 28) . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | No. . | % . | |
| ORR | 13 | 93 | 14 | 100 | 27 | 96 | 14 | 100 | 12 | 86 | 26 | 93 |
| CR | 2 | 14 | 9 | 64 | 11 | 39 | 11 | 79 | 3 | 21 | 14 | 50 |
| PR | 11 | 79 | 5 | 36 | 16 | 57 | 3 | 21 | 9 | 64 | 12 | 43 |
| SD | 1 | 7 | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| PD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7 | 1 | 4 | |
ORR, overall response rate; progressive disease; SD, stable disease.
Pharmacokinetics
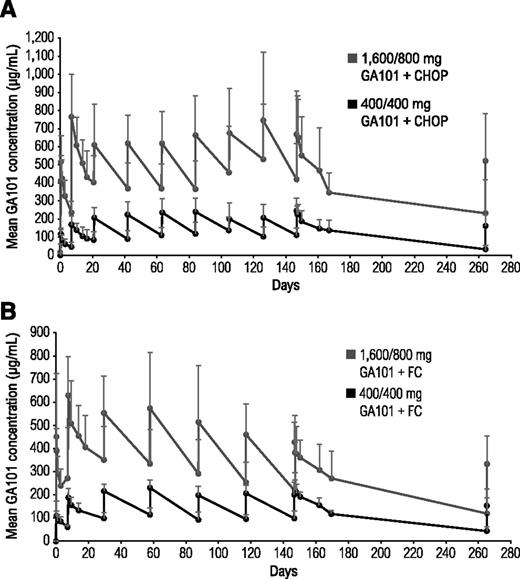
In both cohorts, the 1600/800-mg arms had higher mean obinutuzumab serum concentrations than the 400/400-mg arms (Figure 2). Patients who achieved CR had higher mean serum obinutuzumab concentrations than patients with PR, which remained higher throughout treatment (data not shown).
Mean obinutuzumab (GA101) serum concentrations in combination with chemotherapy. (A) Comparison of two different doses of obinutuzumab plus CHOP and (B) two different doses of obinutuzumab plus FC.
Mean obinutuzumab (GA101) serum concentrations in combination with chemotherapy. (A) Comparison of two different doses of obinutuzumab plus CHOP and (B) two different doses of obinutuzumab plus FC.
Pharmacodynamics
As expected in a relapsed/refractory setting, patients had low baseline B-cell counts in both the G-CHOP (median, 0.066 × 109/L; range, 0 to 0.693 × 109/L) and G-FC cohorts (median, 0.035 × 109/L; range, 0 to 3.463 × 109/L). Overall, B-cell counts fell to <0.04 × 109/L after cycle 1 and did not recover by the cutoff date because most patients were receiving maintenance treatment. Serum immunoglobulin A (IgA), IgG, and IgM levels decreased from baseline through cycle 4 to day 28 of follow-up, but median levels remained normal. No differences in Igs between dose groups were observed in either cohort, but 20% of patients experienced a drop in IgG from normality at baseline to values below the laboratory lower limit of normal; 1 patient (G-FC 1600/800-mg group) with hypogammaglobulinemia required treatment.
Discussion
These data demonstrate the clinical efficacy and acceptable safety profile of obinutuzumab combined with chemotherapy for the treatment of relapsed/refractory FL, supporting previous findings of obinutuzumab monotherapy in this patient setting.18-20 No obinutuzumab-related dose-limiting AEs or unexpected AEs were identified versus obinutuzumab monotherapy.18-20,23 All patients receiving G-CHOP completed treatment. Six patients treated with G-FC discontinued therapy (5 due to AEs). IRRs were the most common AE, which occurred mostly during cycle 1 and were mostly mild or moderate (in each randomized arm, 1 patient had grade 3/4 IRRs). The most frequent grade 3/4 AE in both cohorts was neutropenia, with a higher incidence in the G-CHOP 1600/800-mg and G-FC 400/400-mg arms. Febrile neutropenia was uncommon and consistent with previous studies of patients with relapsed/refractory FL who were administered CHOP or rituximab plus CHOP (R-CHOP).10,24 Infections (all grades) were higher in the 1600/800-mg arm of both cohorts, with grade 3/4 infections occurring in 3 patients in each arm of the G-CHOP cohort and in 4 patients and 1 patient in the 1600/800-mg and 400/400-mg G-FC arms, respectively. SAEs were observed in 29% and 25% of patients treated with G-CHOP and G-FC, respectively. These rates are similar to previous reports of patients with relapsed/refractory FL treated with CHOP or R-CHOP. In the European Organisation for Research and Treatment of Cancer (EORTC) 20981 study, which enrolled patients with FL in first relapse, SAEs were observed in 27% and 30% of patients treated with CHOP and R-CHOP, respectively. In EORTC 20981, 48% and 55% of patients given CHOP and R-CHOP, respectively, experienced grade 3/4 neutropenia,10,24 which is comparable with the 39% incidence observed in the G-CHOP cohort in our study.
No new safety signals were seen in the G-FC arms versus previous trials of rituximab plus fludarabine, cyclophosphamide, and mitoxantrone in patients with relapsed FL or mantle cell lymphoma6 or in patients with FL treated with first-line fludarabine, cyclophosphamide, and rituximab followed by maintenance therapy.25 However, G-FC was less well tolerated than G-CHOP. The less favorable AE profile seen with G-FC may be the consequence of the small sample size, especially since tolerability seemed better for G-FC 1600/800 mg than G-CHOP 400/400 mg, or to differences in the 2 patient populations (G-FC–treated patients were more extensively pretreated, with a longer median time from diagnosis). Yet, FC-related tolerability issues have been previously reported. In the Eastern Cooperative Oncology Group (ECOG) 1496 study comparing FC with cyclophosphamide, vincristine, and prednisone (CVP) in previously untreated patients with indolent lymphoma, recruitment to the FC arm was halted after 8 deaths occurred during induction.26
Obinutuzumab chemotherapy efficacy data are encouraging, and response rates, particularly CR rates, compare favorably with previous reports of rituximab chemotherapy in patients with relapsed disease.6,10,24 In a study of rituximab-naive patients with relapsed/refractory FL who were given R-CHOP, 30% achieved CR at treatment end.24 In GAUDI, in which all but 1 patient had been treated with rituximab, 39% of G-CHOP–treated patients had CRs. Moreover, all 14 patients with rituximab-refractory disease in the GAUDI study responded to treatment, including 4 CRs. A clear dose-response relationship was not apparent in this limited patient series. The CR rate was higher for the G-CHOP 1600/800-mg arm than for the G-CHOP 400/400-mg arm, but the opposite was observed for G-FC. This may have been due to differences in tumor loads: 4 patients in the G-FC 1600/800-mg arm had a sum of the product of diameters >10 000 mm2; all had PRs.
Mean obinutuzumab serum concentrations were higher in both obinutuzumab chemotherapy 1600/800-mg arms compared with the 400/400-mg arms. Regardless of chemotherapy backbone, mean obinutuzumab serum concentrations from cycle 2 onward were higher in patients with CR than those with PR, suggesting that individuals with CR maintain high obinutuzumab serum concentrations and may eliminate obinutuzumab more slowly, possibly reflecting shrinking tumor load.27 Whether obinutuzumab serum concentration is also correlated to baseline tumor bulk has not been investigated because of the small sample size and heterogeneity of the study population. In patients with iNHL administered obinutuzumab monotherapy, the difference in end-of-treatment response rates between the 400/400-mg and 1600/800-mg dose groups was significant (38%; 95% CI, 7.4% to 68.4%).21 These results, in addition to pharmacokinetic and preclinical data suggesting that higher obinutuzumab doses and use of loading doses would rapidly increase and sustain obinutuzumab serum concentrations,28-30 led to the selection of a flat dose of 1000 mg for further clinical trial testing (loading doses on days 1, 8, and 15 of cycle 1; infusions on day 1 of subsequent cycles).
Patients who responded to therapy in the GAUDI study were eligible to receive maintenance therapy. Thus, additional response and time-dependent end points will be reported. On the basis of the currently available data, G-CHOP—regardless of dose—appears to have a safety profile similar to that of R-CHOP, the current standard of care for FL. Consequently, the design of the GAUDI study has been amended to explore the safety of obinutuzumab plus CHOP or bendamustine in 80 treatment-naive patients. Bendamustine is gaining momentum as a treatment option for patients with first-line disease.31 Other trials to evaluate the potential of obinutuzumab in indolent and aggressive B-cell lymphomas are underway: G-FC is being further explored in a phase 1 study (NCT01300247) of patients with previously untreated chronic lymphocytic leukemia, while obinutuzumab 1000 mg plus chemotherapy (CHOP, CVP, or bendamustine) is being compared against rituximab chemotherapy in patients with previously untreated iNHL in the phase 3 GALLIUM study (NCT01332968).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ‘‘advertisement’’ in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Paula Marlton, Mark Hertzberg, Max Wolf, John Catalano, Nicholas Wickham, Andrew Nicol (Australia), Barbara Eichhorst (Germany), Maurizio Martelli (Italy), Antonio Salar (Spain), and Martin Dyer (United Kingdom) for their roles in study design and patient recruitment, and Elina Asikanius for prior statistical support.
Support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
Authorship
Contribution: J.R., A.D., G.C., F.M., G.S., R.M., G.L., and E.W.-F. provided data analysis; J.R., A.D., G.C., F.M., G.S., R.M., U.V., M.W., G.L., and E.W.-F. interpreted data and commented on the manuscript; J.R., A.D., F.M., U.V., M.W., G.L., and E.W.-F. wrote the manuscript; G.C. provided data; G.S. provided study materials; and R.M., M.W., and E.W.-F. designed the trial.
Conflict-of-interest disclosure: J.R. received travel support and accommodation at the 2010 and 2011 American Society of Hematology Annual Meeting and Exposition. A.D. is on Roche advisory boards and has received speaker’s fees, research funding, and a travel grant. G.C. has received speaker’s fees from Roche and GlaxoSmithKline, travel support from Roche, Celgene, and Mundipharma, and has had a consultancy with Roche. F.M. has been a speaker (lectures) and on the advisory boards of Roche, Celgene, Mundipharma, and Spectrum. G.S. has received honoraria from Roche for speaker’s fees and advisory board participation. R.M. has received speaker’s fees and travel grants from Roche/Genentech. U.V. is a member of the Roche global advisory board. M.W., G.L., and E.W.-F. are employees of F. Hoffmann-La Roche Ltd.
Correspondence: Andrew Davies, Cancer Sciences Unit, Southampton General Hospital, Tremona Rd, Southampton SO16 6YD, United Kingdom; e-mail: a.davies@southampton.ac.uk.
References
Author notes
J.R. and A.D. contributed equally to this study.