Key Points
Recurrent U2AF1 mutations are associated with missplicing in the specific genes.
U2AF1 mutant protein might identify the specific sequence signals at the splice sites.
Abstract
Recently, recurrent mutations of spliceosomal genes were frequently identified in myeloid malignancies, as well as other types of cancers. One of these spliceosomal genes, U2AF1, was affected by canonical somatic mutations in aggressive type of myeloid malignancies. We hypothesized that U2AF1 mutations causes defects of splicing (missplicing) in specific genes and that such misspliced genes might be important in leukemogenesis. We analyzed RNA deep sequencing to compare splicing patterns of 201 837 exons between the cases with U2AF1 mutations (n = 6) and wild type (n = 14). We identified different alternative splicing patterns in 35 genes comparing cells with mutant and wild-type U2AF1. U2AF1 mutations are associated with abnormal splicing of genes involved in functionally important pathways, such as cell cycle progression and RNA processing. In addition, many of these genes are somatically mutated or deleted in various cancers. Of note is that the alternative splicing patterns associated with U2AF1 mutations were associated with specific sequence signals at the affected splice sites. These novel observations support the hypothesis that U2AF1 mutations play a significant role in myeloid leukemogenesis due to selective missplicing of tumor-associated genes.
Introduction
Pre-mRNA splicing is one of the vital physiologic functions in eukaryotic gene expression.1 Most human genes are spliced in 2 or more patterns to produce mRNAs that encode protein variants, a process known as physiologic alternative splicing. Alternative exon usage is determined by the selection of splice sites and results in exon skipping or retention. Accordingly, alteration of the exon usage ratio alters the proportion of mRNA isoforms with and without the affected exon. For instance, exon skipping caused by alternative splicing has been found to be altered in various cancers.2 Splicing is carried out by a complicated and dynamic molecular machine known as the spliceosome. Errors in splicing can be a result of somatic mutations of spliceosomal components leading to aberrant and potentially pathological mRNA isoform composition.
Recently, somatic mutations of several spliceosomal proteins (U2AF1, SRSF2, SF3B1) have been identified in myeloid malignancies, in particular myelodysplastic syndromes (MDS), MDS/myeloproliferative neoplasms (MDS/MPN), and secondary acute myeloid leukemia (sAML).3-9 Although these mutations are functionally related through effects on the splicing machinery, the downstream consequences of these mutations may be diverse and involve different oncogenic pathways. These spliceosomal factor mutations are associated with specific pathomorphologic features, clinical phenotypes, and coexisting somatic mutational patterns,10-14 suggesting that downstream consequences of individual mutations may be distinct. SRSF2 mutations are strongly associated with chronic myelomonocytic leukemia,5,6,15 mutations in U2AF1 are more common in advanced myelomonocytic leukemias with poor outcome,4 whereas mutations in SF3B1 are associated with the presence of ring sideroblasts, conveying a comparatively benign prognosis.11 On the basis of the canonical location within the affected spliceosomal gene, these missense mutations are unlikely to be simply hypomorphic, but rather they appear to result in change of function.4,7,16 Here we explore the effects on patterns of alternative splicing due to mutations in the splicing factor U2AF1.
The emergence of spliceosomal mutations as a novel leukemogenesis mechanism raises several questions: what are the critical downstream target genes affected, what is the molecular context of these genes, and how they are specifically targeted by the mutant U2AF1 protein? In this study, we used next-generation genomic platforms to investigate (i) U2AF1 mutant-specific splicing patterns, (ii) specific genes affected by missplicing, and (iii) the coexistence of other molecular defects involving these misspliced genes in cancer.
Methods
Patients
Tumor DNA was obtained from patients’ bone marrow. Informed consent for sample collection was obtained according to protocols approved by the Institutional Review Board and in accordance with the Declaration of Helsinki. Diagnoses of MDS, MDS/MPN, MPN, and sAML were confirmed and assigned according to World Health Organization classification criteria. The clinical characteristics of patients investigated in this study are presented in supplemental Table 3 (available on the Blood website).
DNA sequencing
Selected exons of the U2AF1 gene were amplified and subjected to direct genomic sequencing using standard techniques on the ABI 3730xl DNA analyzer (Applied Biosystems, Carlsbad, CA) as previously described.17-19 Positive mutations were detected by bidirectional sequencing and confirmed using germline DNA obtained from nonclonal CD3+ T cell fraction. Whole exome capture was accomplished on the basis of liquid phase hybridization of sonicated genomic DNA having 150 to 200 bp of mean length to the bait cRNA library synthesized on magnetic beads (SureSelect; Agilent Technology, Santa Clara, CA), according to the manufacturer’s protocol. SureSelect Human All Exon 50Mb kit was used for targeted, exome capture. The captured targets were subjected to massive sequencing using Illumina HiSequation 2000. Generation of .bam files with its preprocessing and detection of somatic point mutations or insertions and deletions was done as previously described.7 Additionally, for detailed analyses, exome sequencing data (n = 197) on AML patients obtained through The Cancer Genome Atlas (TCGA) data portal (https://tcga-data.nci.nih.gov/tcga/) were used.
Whole RNA sequencing
We have used publically available RNAseq data from TCGA data portal for 97 patients (https://tcga-data.nci.nih.gov/tcga). We selected 6 cases harboring U2AF1 mutation (c.101C>T, p.S34F, n = 4, and c.101C>A, p.S34Y, n = 2) for which deep RNAseq20 data were available. We also selected 14 cases that were wild type (WT) for any spliceosomal factor mutation. To further demonstrate specificity of U2AF1 mutations with respect to other spliceosomal mutations (SRSF2, SF3B1, U2AF26), we selected 7 additional cases with mutations in these other spliceosomal factor genes.
Global differential splicing pattern analysis
We quantified exon inclusion ratios based on paired-end RNAseq data. SpliceTrap software (http://rulai.cshl.edu/splicetrap/) was used to quantify the frequency of inclusion of each exon21 and to extract counts of paired end reads that span each exon junction in the genome. For this purpose, each exon was tested for inclusion or exclusion with respect to adjacent exons (supplemental Figure 3). SpliceTrap considers individual exons in whole genomes and is not limited by analysis of known repository of transcripts. This unbiased method is a suitable approach for possible novel discovery of unknown/unexpected splicing variants. Each exon was tested with respect to adjacent exons. Within each triplet, each exon was labeled as A, B, and C, exon B being the one screened for every triplet in the transcriptome. According to this method, we counted reads spanning between exon A/B, B/C, and A/C, where reads spanning the A/C junction reflect the proportion of mRNA missing exon B. The sum of reads between exons A/B and B/C divided by 2 reflects the proportion of mRNA that contains exon B. In order to estimate the frequency of exon B skipping, we divided the number of reads spanning A/C by half of the sum of the reads spanning A/B and B/C. By following these guidelines, we extracted alternative splicing patterns for 20 patients (6 U2AF1 mutant patients and 14 spliceosomal WT patients). Using the frequency of skipped reads to represent the skipping ratio is independent from variation in coverage between different RNAseq samples and is a normalization step itself. The unpaired t test was used to assess the difference of exon usage between these 2 groups. For each exon tested we compared average exon usage between U2AF1 mutants and the WT group, with associated P values generated. Statistical difference of P < .0001 and average difference of ±15% in frequency of exon usage was considered valid for an exon tested. Using this approach, we detected changes in exon skipping (excess of shorter mRNA missing an exon) as well as in exon retention (excess of longer mRNA incorporating an exon) (supplemental Figure 3).
RT-PCR analysis of U2AF1 mutant and WT patients
RNA was extracted from bone marrow or peripheral blood mononuclear cells of patients with and without U2AF1 mutations by TRIzol (Invitrogen, Carlsbad, CA). Reverse transcription–polymerase chain reaction (RT-PCR) was performed using a primer pair specific for detecting exon skipping (exon7; CEP164). We amplified 100 ng of cDNA in 35-cycle RT-PCR reaction at annealing temperature of 60°C. The status of exon skipping or retention was determined by size differences determined by gel electrophoresis.
Sequence analysis of regions around 3′ and 5′ splice site
Sequence information was extracted from adjacent 3′ and 5′ splice site for all exons that were surveyed for differential exon usage. For the 3′ splice sites, sequence was extracted from 20 bp upstream to 3 bp downstream of the intron/exon junction. For the 5′ splice sites, sequence was extracted from 3 bp upstream to 5 bp downstream of the exon/intron junction. Exons were divided into 3 groups according to exon usage levels (exon skipping 0% to 5%, 40% to 60%, and 90% to 100%). All of the splice site sequences (human genome release of 19 hg) were obtained through the table browser available at the University of California Santa Cruz genome browser website (http://www.genome.ucsc.edu/). Exon usage levels were obtained from all WT samples used in this study. Additionally, we extracted sequence information for the genes that were found to have different exon usage level in U2AF1 mutants. These were divided into 2 subcategories: exons that were found to have an excess of exon skipping and exons that had an excess of exon retention. The last group of sequences was a randomly selected set of 1000 exons. Sequence logos were generated using the WebLogo22,23 online application (http://weblogo.berkeley.edu). Sequence logos were used as a graphical representation of overrepresentation of certain nucleotides around 5′ and 3′ splice sites. The overall height of the stack represents sequence conservation at a given position, and the height of symbols representing nucleotides indicates the frequency of a given nucleotide at given position (see Figure 4; supplemental Figure 1). An increased height of any nucleotide is an indication of a higher frequency of the specific nucleotide at that position.
Expression analysis
Expression array data (Affymetrix Human Genome U133 Plus 2.0 Array) were obtained through the TCGA Data Portal (https://tcga-data.nci.nih.gov/tcga). To select low U2AF1 expressor patients, we selected samples that had expression lower than 2 standard deviations from the mean. Expression data for U2AF1 were normally distributed. The statistical difference between normal and low U2AF1 expressors was assessed using an unpaired t test.
Publicly available databases
The February 2009 human reference sequence (GRCh37) produced by the Genome Reference Consortium was used as the reference genome (University of California Santa Cruz genome browser; http://genome.ucsc.edu/cgi-bin/hgGateway). Somatic mutation data were searched by the Catalogue of Somatic Mutations in Cancer database on the Welcome Trust Sanger Institution Website (http://www.sanger.ac.uk/genetics/CGP/cosmic/). Each potential mutation was compared against databases of known single nucleotide polymorphisms (SNPs), including Entrez Gene (http://www.ncbi.nlm.nih.gov/gene) and the Ensemble Genome Browser (http://useast.ensembl.org/index.html).
Cytogenetics and SNP array (SNP-A) analyses
SNP-A assays were processed as previously described.24,25 Affymetrix Human Mapping 250K NSP microarray and Human Genome-Wide SNP Array 6.0 Kit (Affymetrix, Santa Clara, CA) were used. Patients with SNP-A lesions concordant with metaphase cytogenetics or typical lesions known to be recurrent required no further analysis. Germline changes reported in our internal or publicly available (Database of Genomic Variants; http://projects.tcag.ca/variation) copy number variation databases were considered nonsomatic and excluded from further analysis. Results were obtained using Copy Number Analyzer for Affymetrix GeneChip (version 3.0)26 (Affymetrix Human Mapping 250k NSP microarray kit) or Genotyping Console (Affymetrix Genome-Wide SNP Array 6.0 kit). All other lesions were additionally confirmed as somatic or germline by analysis of CD3-sorted cells.27
Statistical analysis
For comparison of the exon usage levels between groups, with and without U2AF1 mutations, statistical analysis was performed using the described workflow. We used 201 837 exons/variables and 20 observations (n = 6 U2AF1 mutants and n = 14 U2AF1 WT). The probability that a particular score would occur by chance was assessed using permutation testing and a random model.28 We used randomization-based significance testing. This leads to the notion of normalized scores, expressed as the number of standard deviations from the mean of the random distribution. The returned P values were ≥5 standard deviations from expected P value of a random set (Student t test was used to generate the P values). That allowed one to reject the null hypothesis of there being no difference between the 2 cohorts tested.
Results
Detection of U2AF1 mutations and genotypic associations
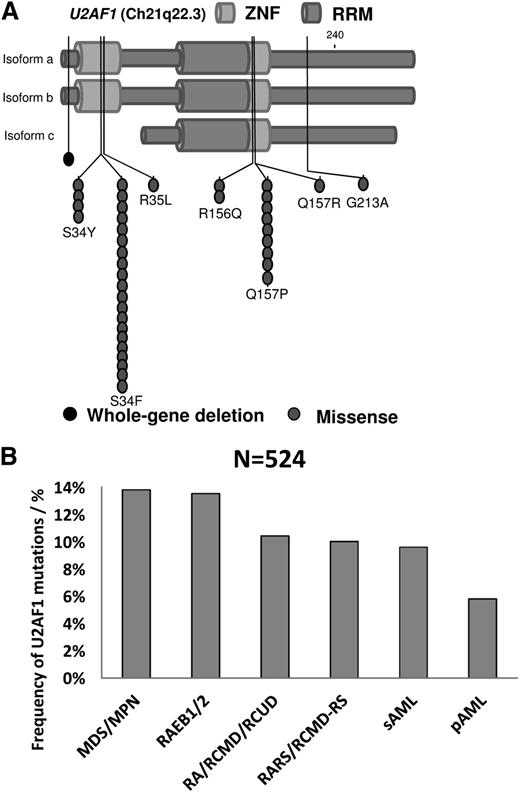
We examined the U2AF1 mutational status of a cohort of patients (n = 524) with various hematologic malignancies, including MDS, MDS/MPN, MPN, and AML using Sanger sequencing and Next Generation Sequencing exome sequencing to identify cases for further analysis. Of these, 46 cases harboring heterozygous somatic mutations in U2AF1 (9%) were found (Figure 1). Overall, U2AF1 mutations were more frequently present in male patients than in female patients (83% vs 17%, P < .001) and were nearly evenly distributed among patients with AML (7%, in both sAML as well as primary AML), MDS (10%), MPN (8%), MDS/MPN, (14.5%), and MDS with higher risk (12.5%). There were 8 distinct missense mutations, including A26V (n = 1), R35L (n = 1), S34Y (n = 4), S34F (n = 21), R156Q (n = 2), Q157P (n = 13), Q157R (n = 3), and G213A (n = 1) (supplemental Table 1). The 2 most frequent mutations, S34F(47%) and Q157P(29%), accounted for more than 75% of all mutations detected. Almost invariably (97.8%), the mutations were localized in 1 of the 2 zinc finger domains (Figure 1A).
Distribution and frequency of U2AF1 mutations across gene domains and different hematological malignancies. (A) Three isoforms of U2AF1 are shown with the 2 zinc finger domains (ZNF) and the RNA recognition motif (RRM) highlighted. Almost all identified U2AF1 missense mutations are located in 1 of the 2 ZNF domains. (B) Comparison of the frequency of U2AF1 mutations between different hematological malignancies. MDS/MPN and high-risk MDS (RAEB1/2) showed the most frequent mutations (14% and 13%, respectively), whereas primary AML showed the least (6%). RA, refractory anemia; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-RS, refractory cytopenia with multilineage dysplasia with ring sideroblasts; RCUD, refractory cytopenia with unilineage dysplasia.
Distribution and frequency of U2AF1 mutations across gene domains and different hematological malignancies. (A) Three isoforms of U2AF1 are shown with the 2 zinc finger domains (ZNF) and the RNA recognition motif (RRM) highlighted. Almost all identified U2AF1 missense mutations are located in 1 of the 2 ZNF domains. (B) Comparison of the frequency of U2AF1 mutations between different hematological malignancies. MDS/MPN and high-risk MDS (RAEB1/2) showed the most frequent mutations (14% and 13%, respectively), whereas primary AML showed the least (6%). RA, refractory anemia; RCMD, refractory cytopenia with multilineage dysplasia; RCMD-RS, refractory cytopenia with multilineage dysplasia with ring sideroblasts; RCUD, refractory cytopenia with unilineage dysplasia.
Mutational screening detected concomitant mutations in DNMT3A, RAS family genes (KRAS/NRAS), ASXL1, RUNX1, TET2, CBL, and IDH family genes (IDH1/2) in 25%, 25%, 24%, 13%, 11%, 12%, and 5% of patients, respectively (supplemental Figure 2). There was only 1 case (refractory anemia with ring sideroblasts, trisomy 8) harboring a double U2AF1/SF3B1 spliceosomal mutation. Mutations in a different spliceosomal factor, SRSF2, were mutually exclusive in our cohort. Additionally, 11 out of 40 patients did not harbor any additional mutations from the panel of genes tested (supplemental Figure 2).
Functional importance of U2AF1 mutants
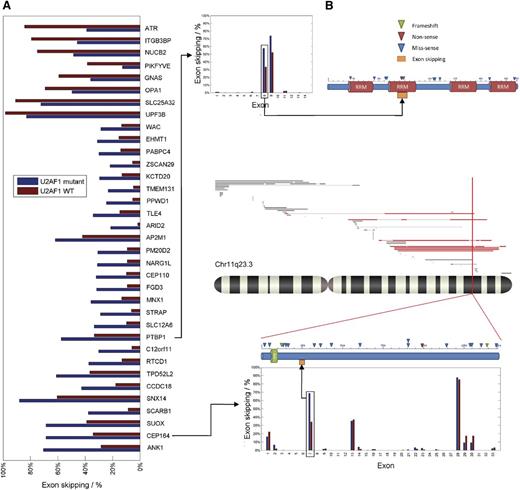
To understand the functional consequences of U2AF1 mutations, we studied mutation-specific exon usage patterns as determined by deep RNA sequencing of U2AF1 mutant (n = 6) and WT (n = 14) cases. Using t test (P < .0001), average and absolute difference in exon usage ratio (more than ±15%) as criteria, we successfully tested 201 837 exons in 17 097 genes. Using this approach, we found 35 exons in 35 genes with a significantly altered pattern of inclusion or exclusion in U2AF1 mutant cases in comparison with spliceosomal WT cases (Figure 2). The U2AF1 mutant-specific splicing patterns were categorized into 2 groups: exon skipping (lower exon usage) or exon retention (higher exon usage) with respect to WT (supplemental Figure 3). Most (77%) of the significantly altered exons showed more exon skipping in patients carrying U2AF1 mutation. The rest (23%) represented increased exon retention patterns. Among the genes exhibiting differential exon usage patterns, we identified genes involved in different stages of mitosis (CEP164, EHMT1, WAC, and ATR). Another distinct group of genes identified consists of genes involved in RNA processing (PTBP1, STRAP, PPWD1, PABPC4, and UPF3B) (Figure 2).
Differences of exon usage frequencies in genes that were identified. Exon skipping frequencies were based on RNAseq data, averaged and presented as bar graphs. (A) Bars in dark blue represent U2AF1 mutants; dark brown bars represent WT. The order of genes was determined using the average difference between U2AF1 mutant and WT exon skipping frequency. (B) Detailed frequency of exon skipping of all exons screened for PTBP1 (upper panel) and CEP164 (lower panel). Additional mutational information is depicted for both selected genes. CEP164 lower panel contains additional SNP karyotyping data depicting samples that had the CEP164 locus deleted (highlighted in red).
Differences of exon usage frequencies in genes that were identified. Exon skipping frequencies were based on RNAseq data, averaged and presented as bar graphs. (A) Bars in dark blue represent U2AF1 mutants; dark brown bars represent WT. The order of genes was determined using the average difference between U2AF1 mutant and WT exon skipping frequency. (B) Detailed frequency of exon skipping of all exons screened for PTBP1 (upper panel) and CEP164 (lower panel). Additional mutational information is depicted for both selected genes. CEP164 lower panel contains additional SNP karyotyping data depicting samples that had the CEP164 locus deleted (highlighted in red).
To confirm the aberrant pattern of alternative splicing in U2AF1 mutants, we amplified cDNA containing a specific exon found to be alternatively skipped on the basis of RNAseq reads. As an example, we selected CEP164 exon 7, which was observed to be most frequently skipped in U2AF1 mutants in comparison with cases with WT U2AF1. Using primers in exon 6 and exon 8, skipping of exon 7 yielded a 220-bp product, whereas inclusion of exon 7 yielded a 298-bp product (Figure 3C). As predicted by the RNAseq results, only the exon 7–skipped product was observed in U2AF1 mutant cases, whereas in 6 out of 7 U2AF1 WT cases, both 298-bp and 220-bp bands were detected, suggesting that exon 7 was partially skipped. These findings were further confirmed by using primers in exon 6 and exon 7, in which amplification products were detected only in the WT U2AF1 cases, but not in the U2AF1 mutant cases (Figure 3C).
Transcriptional analysis of patients with splicing factor mutations. (A) Comparison of levels of U2AF1 mRNA between U2AF1 mutants, WT cases, and WT cases with low expression of U2AF1 (red, blue, and green colors, respectively). The mean expression level is indicated by the dashed line. (B) Exon skipping levels in 3 genes comparing U2AF1 mutants, WT, and WT with low expression levels (red, blue, and green bars, respectively). (C) Validation of RNAseq results on exon 7 of the CEP164 gene using an independent set of patients by RT-PCR. (D) Comparison of exon skipping levels between patients bearing mutations in different spliceosomal factors: SF3B1, SRSF2, U2AF26, and U2AF1. ATR, ataxia telangiectasia and Rad3-related.
Transcriptional analysis of patients with splicing factor mutations. (A) Comparison of levels of U2AF1 mRNA between U2AF1 mutants, WT cases, and WT cases with low expression of U2AF1 (red, blue, and green colors, respectively). The mean expression level is indicated by the dashed line. (B) Exon skipping levels in 3 genes comparing U2AF1 mutants, WT, and WT with low expression levels (red, blue, and green bars, respectively). (C) Validation of RNAseq results on exon 7 of the CEP164 gene using an independent set of patients by RT-PCR. (D) Comparison of exon skipping levels between patients bearing mutations in different spliceosomal factors: SF3B1, SRSF2, U2AF26, and U2AF1. ATR, ataxia telangiectasia and Rad3-related.
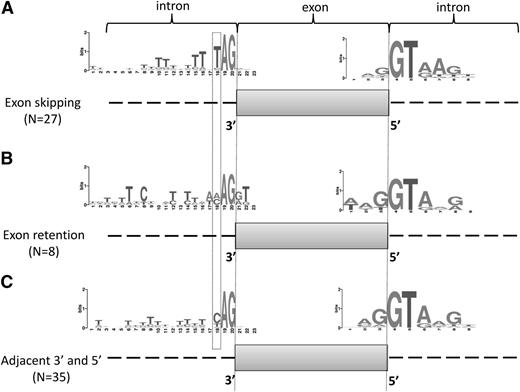
Frequencies of nucleotides surrounding 39 and 59 splice sites adjacent to exons affected by U2AF1 mutations. Exons that were more often skipped (A) or more often retained (B) in U2AF1 mutants were combined into 2 groups, and the splice site consensus sequences were derived. Adjacent splice sites were analyzed as a control set (C). Nucleotide frequencies are represented using WebLogo software. The height of each stack represents the information content of that position in bits. The height of each letter represents the frequency of occurrence of each nucleotide.
Frequencies of nucleotides surrounding 39 and 59 splice sites adjacent to exons affected by U2AF1 mutations. Exons that were more often skipped (A) or more often retained (B) in U2AF1 mutants were combined into 2 groups, and the splice site consensus sequences were derived. Adjacent splice sites were analyzed as a control set (C). Nucleotide frequencies are represented using WebLogo software. The height of each stack represents the information content of that position in bits. The height of each letter represents the frequency of occurrence of each nucleotide.
Transcriptional analysis of U2AF1 WT patients with low U2AF1 expression
To better understand the functional role of the U2AF1 mutations, we also compared exon usage levels in patients with low expression of U2AF1. If the U2AF1 mutations were simply hypomorphic, one would expect to find similar missplicing patterns in patients with low levels of U2AF1 expression and those with mutant U2AF1. We focused our analyses on exon usage of genes that were identified to have differential splicing patterns between U2AF1 mutant and WT cases (Figure 3A). Low expressors of U2AF1 had similar (P > .05) usage levels (less than ±10%) as did those of WT in 19 exons (55%), similar usage to U2AF1 mutant in 1 exon (3%), and intermediate exon usage to WT and mutant in 14 exons (42%) (Figure 3B). This result indicates that low expression of U2AF1 does not create the same aberrant splicing patterns observed in U2AF1 mutants. Additionally, all the exons of 35 genes that were differentially spliced in U2AF1 mutant were screened, but no significant differences were found.
Comparison of splicing patterns in U2AF1 mutant patients with other spliceosomal factor mutations
Although mutations in several different splicing factors have been described in myeloid neoplasms, each factor seems to have a unique distribution and different effects on survival. This suggests that the target genes might be different for each mutant factor. To investigate this idea, we selected cases with somatic mutations in the splicing factors SF3B1, SRSF2, and U2AF26 and compared the splicing pattern of representative genes to our U2AF1 mutant cases. As is shown in Figure 3D, each factor mutation was associated with different changes in the splicing patterns of these genes. The dotted lines correspond to the level of exon skipping seen in WT cases.
Sequence signatures of splice sites associated with missplicing in U2AF1 mutants
To explain differences in exon usage between U2AF1 mutants and WT, we analyzed the splice site sequences surrounding all the exons analyzed in this study. Figure 4 shows the sequence patterns flanking the alternative exons for the subset that showed increased exon skipping (Figure 4A), the subset that showed increased exon retention (Figure 4B), and the flanking exons (Figure 4C). The splice site sequence patterns generally match the consensus sequences for 3′ and 5′ splice sites with the exception of the −3 nucleotide in relation to the 3′ intron/exon junction (boxed position 16 in Figure 4). This position had a higher frequency of thymidine (83%) adjacent to exons that were skipped more frequently in U2AF1 mutants and a very low frequency of thymidine adjacent to exons that were more frequently included in U2AF1 mutants. The consensus of all 3′ splice sites shows a nearly equal probability of a thymidine or a cytosine at this position, as is seen in Figure 4C. As discussed below, this position is immediately adjacent to the AG dinucleotide that is known to be bound by U2AF1. To determine whether this sequence signature was related to the frequency of skipping of the adjacent exon, we bound all alternative exons by their skipping frequencies and analyzed the splice sites sequences (supplemental Figure 1). All subsets of alternative exons had a very similar pattern of C and T at position 16 that matches the overall consensus sequence.
Mutations and deletions in the misspliced genes by U2AF1 mutants
To further explore the common pathophysiology between exon usage alteration in U2AF1 mutants and other somatic molecular events occurring in myeloid neoplasms, we searched the genes in which excess exon skipping or retention was detected for mutations and deletions. Out of 35 genes misspliced in U2AF1 mutants, deletion of the corresponding locus was observed in 34 genes (97%), and somatic mutations were observed in all 35 (100%) genes (supplemental Table 2). Remarkably, some of these somatic mutations were located in the exact exons for which exon usage was changed by U2AF1 mutation. For example, 2 missense and 1 nonsense mutation were observed in the same RNA recognition motif (RRM) domain of PTBP1, which was skipped more frequently in U2AF1 mutant cases (Figure 2). In another example, the CEP164 locus, in which exon 7 was highly skipped, was frequently deleted (chr11q23.3) in myeloid malignancies (supplemental Table 2), whereas in a solid tumor cohort, missense/nonsense/frameshift mutations were reported as well (Catalogue of Somatic Mutations in Cancer database).
Discussion
Recently, frequent recurrent U2AF1 mutations in myeloid malignancies were reported to result in splicing alteration, which causes exon skipping or less expression due to unspliced pre-mRNA.3,4,7 In this study, we identified distinct mutation-specific exon usage patterns as the functional consequences of U2AF1 mutations. U2AF1 mutations are associated with abnormal splicing of genes involved in functionally important pathways, including cell cycle progression and RNA processing. Moreover, some of these genes are somatically mutated or deleted in various cancers. Of note is that missplicing patterns associated with U2AF1 mutations were observed in exons flanked by a characteristic splice site sequence bias. These findings supply novel information on how the recurrent U2AF1 mutations might participate in the pathophysiology of myeloid malignancies.
In this study, deep RNA sequencing of U2AF1 mutant cases showed significant alterations of splicing patterns in multiple genes. Functionally related gene groups were affected by missplicing due to U2AF1 mutations. For example, genes involved in different stages of mitosis (CEP164, EHMT1, WAC, and ATR) or in RNA processing (PTBP1, STRAP, PPWD1, PABPC4, and UPF3B) were affected. Another affected gene, CEP164, is one of the centrosomal proteins involved in G2/M checkpoint control and nuclear divisions.29,30 In various malignancies, the CEP164 locus is frequently deleted or affected by missense/nonsense/frameshift mutations. Thus, the CEP164 locus demonstrates 3 different types of loss of function: deletion, mutation, and splicing defects due to U2AF1 somatic mutations. Of note is that CEP164 and ATR proteins interact with each other in the DNA damage-signaling cascade,30 and ATR is one of the genes frequently mutated in myeloid malignancies. These findings indicate that molecular events due to splicing defects and somatic mutation/deletion might be leukemogenic events via common gene targets.
One of the misspliced genes found here, PTBP1, is known to regulate alternative splicing events through interactions with pyrimidine-rich RNA sequences.31 PTBP1 may also inhibit the binding of U2 snRNP to certain pre-mRNAs, indicating that PTBP1 could be in the same complex as U2AF1 or competing with it.32 Splicing regulatory genes (for example, PTBP1) misspliced by U2AF1 mutations might indirectly promote additional splicing defects. Interestingly, the shorter spliced variant of PTBP1 that is overproduced in U2AF1 mutant cases is missing the second quasi- RRM domain, which is functionally associated with RNA binding.33 Moreover, in solid tumors, somatic mutations were reported in this RRM domain.34-37 These findings suggest that U2AF1 mutations might modify the isoforms of other spliceosomal proteins (for example, PTBP1) by changing splicing pattern or that other spliceosomal genes modified by U2AF1 mutations could indirectly promote other splicing defects as well as the direct effects of U2AF1 mutations.
The U2AF1 protein is part of the heterodimeric U2 auxiliary factor (U2AF) along with the U2AF2 protein. U2AF2 binds to the polypyrimidine tract upstream of the 3′ splice junction, whereas U2AF1 binds to the invariant AG dinucleotide at the 3′ splice junction. The binding of the U2AF complex to the 3′ splice site is one of the early steps in spliceosome formation. There is evidence that the requirement of U2AF1 differs among 3′ splice sites, suggesting that it can serve a regulatory role in alternative splicing decisions. This theory is supported by the finding that most mutations detected by us and other groups are located in either of the 2 zinc finger domains, which are likely involved in RNA binding.3,4,7
Further support comes from our finding of a unique sequence feature in 3′ splice sites affected by U2AF1 mutations. The identity of the nucleotide immediately upstream of the 3′ splice site AG appears to regulate how well the adjacent exon is spliced in the U2AF1 mutants. Normally, this nucleotide is either a T or C and was not thought to be recognized by U2AF1. It now appears that this nucleotide is recognized differently by the mutant U2AF1 in comparison with the WT U2AF1. The molecular basis of this altered recognition is under investigation.
Mutations in myeloid neoplasms of each component of the spliceosome are almost always mutually exclusive,4,7 even if the proteins cooperate with each other in splicing. This implies that defects in these different genes might contribute to modifying spliceosomal function in a unique way and that U2AF1 and other spliceosomal mutations cannot occur in a cumulatively synergistic way. Furthermore, the spectrum of mutations in these genes suggests that they are not simply loss of function alleles but rather have altered functions. To support this theory, we showed that patients with low expression of U2AF1 revealed a splicing pattern similar to that of WT but different from U2AF1 mutant. Another explanation of distinct splicing pattern observed in U2AF1 mutant is that low expressors of U2AF1 might just induce compensatory mechanism on a spliceosomal machinery. Further investigation of this observation is needed to clarify the mechanism.
Clinically, in our cohort, we find that U2AF1 mutations are more frequent in more proliferative phenotypes, including MDS/MPN and high-risk MDS, which require a new therapeutic strategy. Previous reports also showed that U2AF1 mutations are associated with high incidence of leukemic evolution and poor prognosis.3,4,7 In younger patients, more intensive chemotherapy or stem cell transplantation will be indicated in cases with U2AF1 mutations. In elderly patients, more specific drug therapy should be applied, for example, molecular-targeted therapy. In this study, we identified downstream splicing defects in several genes that are functionally important in various cancers. Such molecules could be proposed as novel therapeutic targets in U2AF1 mutant cases.
Our RNA sequencing analysis was applied to the most comprehensive splice sites in coding regions, which provided us with completely novel findings associated with prevalent U2AF1 mutations. Despite the inability to remove false-positive risk thoroughly, genetically reproducible splicing patterns were identified in functionally important genes. Null-model comparisons were a more reasonable statistical methodology in this study than were multiple testing corrections. Further basic experiments, for example, conditional knock-in mutant animal models, will clarify the detail of pathophysiological significance of splicing defects in myeloid neoplasms with various types of spliceosome gene mutations.
In summary, our study validates the change-of-function nature of U2AF1 mutations and describes a set of significantly misspliced genes, functionally correlated, and almost invariably affected by a concomitant molecular alteration, establishing a novel mechanism of leukemogenesis of myeloid malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The results published here are in part based upon data generated by The Cancer Genome Atlas pilot project established by the National Cancer Institute and the National Human Genome Research Institute. Information about TCGA and the investigator and institutions that constitute the TCGA research network can be found at http://cancergenome.nih.gov.
This work was supported by National Institutes of Health (Bethesda, MD) grants RO1-GM104059 (National Institute of General Medical Sciences; to R.A.P.), RO1HL-082983 (National Heart, Lung, and Blood Institute; to J.P.M.), U54 RR019391 (National Center for Research Resources; to J.P.M.), and K24 HL-077522 (National Heart, Lung, and Blood Institute; to J.P.M.); a grant from the AA & MDS International Foundation (Rockville, MD); the Robert Duggan Charitable Fund (Cleveland, OH; to J.P.M.); and a Scott Hamilton CARES grant (Cleveland, OH; to H.M.).
Authorship
Contribution: B.P. and H.M. designed research, performed research, collected data, performed statistical analysis, and wrote the manuscript; K.G. collected data; A.J. and M.A.S. interpreted data and wrote the manuscript; R.P. designed research, contributed analytical tools, collected data, and analyzed and wrote the manuscript; and J.P.M. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hideki Makishima, Taussig Cancer Institute/R40, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: makishh@ccf.org.