To the editor:
Immune responses directed against epitopes derived from the mutated region of nucleophosmin 1 (NPM1) by NPM1mut-specific CD8(+) cytotoxic T cells (CTLs) might be involved in the rejection of NPM1mut myeloid leukemic blasts. NPM1 mutations are one of the most frequent molecular alterations in acute myeloid leukemia (AML) and are an important prognostic marker.1 The mutations cause an abnormal shift of the NPM1 protein from the nucleus to the cytoplasm, described by Falini et al.2 AML patients with NPM1mut, but without FLT3 internal tandem duplication (ITD) mutation, show improved overall survival.3 NPM1mut/FLT3-ITD–negative patients do not seem to benefit from allogeneic stem cell transplantation in first-line treatment; however, this issue is still under evaluation, further clinical trials are ongoing, and also minimal residual disease (MRD) has to be considered in treatment decision.3,4 The functional role of NPM1mut for the improved clinical outcome is still under evaluation. Immune responses to NPM1mut may contribute to favorable prognosis of this AML subtype. Recently, we described specific T-cell responses of CD4+ and CD8+ T cells against epitopes derived from mutated regions of NPM1.5 Two NPM1mut-derived peptides, called #1 and #3, induced specific T-cell responses in patients with NPM1mut (33% and 44%, respectively). NPM1mut AML patients showed a significantly higher frequency of CTL responses against peptide #3 compared with healthy volunteers (P = .046).5 Several leukemia-associated antigens (LAAs) have been defined, most importantly RHAMM, Proteinase 3, and Wilms’ tumor antigen 1 (WT-1). These antigens were tested in clinical peptide vaccination trials.6 Immunologic and clinical responses were detected in patients with different hematologic malignancies.7,8 Berneman et al9 discussed NPM1mut as a further important LAA to attack AML and leukemic stem cells by autologous T cells.
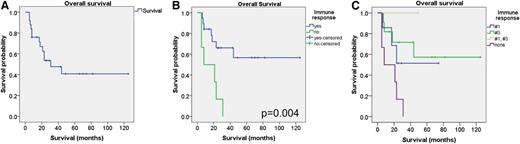
In this work, we performed survival analysis of 25 NPM1mut patients (Figure 1A), analyzed by enzyme-linked immunospot comparing cases with or without specific T-cell responses. Our data suggest a better overall survival of patients with specific CTL responses against peptide #1 or #3 (P = .004; Figure 1B). Immune responses seem to differ in dependence on the epitopes (P = .026; Figure 1C), although this finding has to be interpreted with caution due to the low number of patients. The survival rate of all NPM1mut patients is lower due to other molecular alterations (like FLT3-ITD in 11 of 25 NPM1mut patients) and the inclusion of elderly patients (7 of 25 were older than 60 years of age). Due to its exquisite specificity in leukemia, NPM1mut might constitute an ideal target structure for individualized immunotherapeutic approaches. Analysis with material from larger controlled clinical trials has to be performed. Nevertheless, these data suggest that immune responses might contribute to the clinical outcome. Therefore, immunotherapeutic approaches present a promising strategy for NPM1mut patients for maintenance treatment or with persistent MRD. In an AML patient with NPM1mut and molecular relapse, we demonstrated polyspecific CTL responses against several known LAAs, also NPM1 #3, after preemptive donor lymphocyte infusion.10 Importantly, the immune responses against LAAs were associated with MRD negativity.10 Such persistent responses against NPM1mut epitopes provide a rationale for the development of preemptive maintenance strategies in AML patients with NPM1mut.
Survival analysis of NPM1mut patients. (A) Kaplan Meier plot with the survival analysis of 25 NPM1mut patients. (B) Overall survival of patients with specific CTL responses against peptides #1 or #3. Blue, patients with an immune response; green, patients without any specific CTL response. (C) Overall survival in dependence on the specific epitope. Blue, peptide #1; green, peptide #3; yellow, peptides #1 and #3; purple, no peptide.
Survival analysis of NPM1mut patients. (A) Kaplan Meier plot with the survival analysis of 25 NPM1mut patients. (B) Overall survival of patients with specific CTL responses against peptides #1 or #3. Blue, patients with an immune response; green, patients without any specific CTL response. (C) Overall survival in dependence on the specific epitope. Blue, peptide #1; green, peptide #3; yellow, peptides #1 and #3; purple, no peptide.
Taken together, NPM1mut might constitute an interesting target structure for individualized immunotherapeutic approaches in NPM1mut AML patients. We hypothesize that immune responses against mutated NPM1 may contribute to the favorable prognosis.
Authorship
Acknowledgment: This research was supported by grants from the German Research Foundation (DFG GR2676/3-1).
Contribution: J.G. designed the research, performed research, analyzed and interpreted data, and wrote the paper; V.S. performed research and analyzed and interpreted data; M.S. discussed the data; M.G. performed research and analyzed and interpreted data; K.D. and M.W. analyzed and interpreted data; H.D. discussed the data; and S.H. designed the research, performed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jochen Greiner, Department of Internal Medicine III, University of Ulm, Albert-Einstein-Allee 23, Ulm 89081, Germany; e-mail: jochen.greiner@uniklinik-ulm.de and Department of Internal Medicine, Diakonie-Klinikum Stuttgart, Rosenbergstrasse 38, 70176 Stuttgart; e-mail: greiner@diak-stuttgart.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal