Key Points
A calcineurin-like phosphatase dephosphorylates annexin A2 in the course of cAMP-induced Weibel-Palade body exocytosis.
Dephosphorylation at serine 11 of annexin A2 triggers complex formation with S100A10 that is required for von Willebrand factor secretion.
Abstract
The large multimeric glyocoprotein von Willebrand factor (VWF) is a crucial component of both primary and secondary hemostasis. It is stored in secretory granules of vascular endothelial cells, the Weibel-Palade bodies (WPBs), and is released following stimulation by agonists that raise intracellular Ca2+ or cyclic adenosine monophosphate (cAMP) levels. cAMP-induced exocytosis of WPBs requires protein kinase A activity, but downstream factors that are regulated by phosphorylation/dephosphorylation are not known. Here we identify the complex consisting of the lipid-binding protein annexin A2 (AnxA2) and S100A10 as such a factor. Knockdown and specific rescue approaches reveal that a functional AnxA2-S100A10 complex is required for the forskolin-induced, cAMP-dependent release of VWF. Forskolin triggers dephosphorylation of AnxA2 that is mediated by a calcineurin-like phosphatase and stabilizes the AnxA2-S100A10 complex, thereby promoting VWF release. Serine 11 of AnxA2 was identified as the target residue of this phosphorylation switch because a phosphomimicking mutation at this site prevents complex formation with S100A10 and, in contrast to wild-type or S11A-AnxA2, is unable to restore cAMP-dependent VWF secretion in AnxA2-depleted cells. Thus, complex formation of AnxA2 with S100A10 is a central regulatory mechanism in the acute release of VWF in response to cAMP-elevating agonists.
Introduction
von Willebrand factor (VWF) is a large, adhesive glycoprotein that plays pivotal roles in hemostasis. It mediates platelet adhesion to the vascular wall, triggers platelet activation, and binds to and stabilizes clotting factor VIII.1 Within endothelial cells, VWF is stored in rod-shaped, exocytotic organelles called Weibel-Palade bodies (WPBs).2,3 Exocytosis of WPBs is stimulated by a varied set of agonists that can be separated into 2 main groups: those acting by elevating the free concentration of cytosolic Ca2+ and those acting by raising cyclic adenosine monophosphate (cAMP) levels.4,5 For instance, histamine and thrombin have been shown to mediate secretion of WPBs by increasing the levels of cytosolic free Ca2+,6,7 whereas release of WPBs induced by secretagogues such as epinephrine or forskolin involves cAMP-dependent signaling.8,9 Significant differences exist between Ca2+- and cAMP-evoked WPB exocytosis and, whereas several cellular factors participating in the Ca2+-regulated secretion of WPBs have been identified, much less is known about the molecular mechanisms underlying the cAMP-dependent exocytosis of WPBs.
Different secretagogues can elicit different WPB dynamics.10 Secretion induced by cAMP-raising agents involves the release of only peripheral granules and is accompanied by a protein kinase A (PKA)-dependent perinuclear clustering of the more central granules.11 In contrast, Ca2+-raising agents mediate release of both central and peripheral granules, suggesting differences in the mechanisms of release of WPBs between the 2 pathways. On the other hand, the finding that the small GTPase RalA and the phosphatidic acid–producing enzyme phospholipase D1 are crucial components of both Ca2+- and cAMP-dependent VWF release mechanisms suggests that there is a convergence or interconnection between the 2 pathways some point downstream of the respective agonist and intracellular second messenger.12-15 The point of this convergence and more general aspects of a cross-talk between the Ca2+- and cAMP-dependent pathways initiating WPB exocytosis are not known. Furthermore, although PKA activity is required downstream of cAMP in the regulation of WPB exocytosis,16,17 targets of its activity that are controlled by a phosphorylation/dephosphorylation switch in the course of WPB exocytosis remain to be identified.
Annexins are Ca2+- and phospholipid-binding proteins that are involved in Ca2+-dependent and Ca2+-independent membrane organization and trafficking events.18 Annexin A2 (AnxA2) is a member of the family that can exist either as a monomer or as a heterotetramer, in which 2 monomers bind to a dimer of the EF hand protein S100A10.19 We have shown previously that depleting endothelial cells of AnxA2 or S100A10 or functionally interfering with AnxA2-S100A10 complex formation results in a marked inhibition of histamine-evoked and Ca2+-dependent secretion of VWF from endothelial cells.20 AnxA2-S100A10 also participates in a number of other exocytotic and endocytotic events, for example, the Ca2+-dependent secretion of chromaffin granules21 and the traffic of certain plasma membrane-resident ion channels.22 Interestingly, protein kinase C (PKC)-dependent phosphorylation and PKA-dependent dephosphorylation of AnxA2 appear to regulate complex formation with S100A10,23,24 suggesting an elegant mechanism for regulating activities that require a fully assembled AnxA2-S100A10 complex. These findings prompted us to investigate whether the AnxA2-S100A10 complex plays a role in WPB exocytosis that occurs in response to cAMP-elevating agents.
We show that the AnxA2-S100A10 complex indeed serves an important function in the cAMP-driven secretion of VWF. Forskolin, a cAMP-elevating agonist, concomitantly with triggering VWF secretion causes a calcineurin-mediated dephosphorylation on serine residues of AnxA2 in primary human endothelial cells (HUVECs). Depletion of both AnxA2 and S100A10 leads to a marked inhibition of forskolin-induced VWF secretion, and in AnxA2-depleted cells this defect can be rescued by re-expression of WT AnxA2 but not a phosphomimicking mutant characterized by an Asp for Ser replacement at amino acid 11. Ectopic expression of this S11D-AnxA2 phosphomimicking mutant reveals that a negative charge at Ser-11 inhibits AnxA2-S100A10 complex formation. These results demonstrate that elevation of intraendothelial cAMP induces dephosphorylation of AnxA2 at Ser-11 and thereby a stabilization of the AnxA2-S100A10 complex that in turn is required for efficient VWF secretion.
Methods
The experimental details for antibodies, plasmids, cell culture, transfection, small interfering RNAs (siRNAs), quantification of AnxA2 and S100A10 knockdown and rescue efficiency, and immunoprecipitation are described in supplemental Methods on the Blood website.
Quantification of VWF secretion and drug treatment
HUVECs grown to confluency on collagen-coated 24-well plates were serum-starved overnight. Exocytosis of WPBs was then induced by treatment with 200 µL agonist-containing stimulation medium (basal medium containing 0.5% bovine serum albumin, 100 µM histamine, or 20 µM forskolin/100 µM 3-Isobutyl-1-methylxanthine (IBMX), respectively) at 37°C for 20 minutes, and the amount of secreted VWF was quantified as described.25 Control experiments used 0.5% bovine serum albumin in basal medium without secretagogues to measure the levels of constitutive exocytosis.
Cell loading with 10 µM H-89 (Sigma-Aldrich), 20 µM 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) (Molecular Probes, Eugene, OR), or 50 nM FK-506 (Merck, Darmstadt, Germany) in basal medium was achieved by preincubation for 20 minutes at 37°C for the former two and for 5 hours at 37°C for the latter. All drugs were added from stock solutions prepared in dimethylsulfoxide (DMSO), and drug-free control experiments included the respective amount of solvent (DMSO) in the preincubation medium. After a medium change, cells were incubated for 20 minutes in stimulation medium containing the respective secretagogue, and secretion was analyzed as described above.
Analysis of intracellular AnxA2-S100A10 interactions
To analyze within cells the complex formation of different AnxA2 derivatives with S100A10, we made use of a chimeric protein comprising the N-terminal S100A10 binding sequence of AnxA2 (residues 1 to 18) fused to S100A10. This so-called XM construct clusters S100A10 and AnxA2 derivatives into larger aggregates provided that they are capable of binding one another through the N-terminal AnxA2 sequence.26 The XM construct was transfected into Mardin Darby canine kidney-tetracycline controlled transactivator (MDCK-tTA) cells expressing the Tet-Off transactivator (Clontech). Clones positive for the XM plasmid were selected by G-418 resistance and kept in Dulbecco’s modified Eagle medium containing 10% fetal calf serum, 2 mM l-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 3 µg/mL tetracycline. Expression of the chimeric XM protein was then induced by cultivation in the same medium without tetracycline for 3 days. XM-induced clusters were visualized by immunofluorescence with a monoclonal anti-S100A10 antibody (H21) recognizing the S100A10 part of the XM construct. The interaction of different AnxA2 mutants with S100A10 was tested by analyzing their recruitment to the XM-induced clusters. Therefore, MDCK-tTA-XM cells were induced to express XM-AnxA2 by tetracycline deprivation and then transfected with different green fluorescent protein (GFP)-tagged AnxA2 constructs using Lipofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Subsequently, colocalization of XM-AnxA2 and the different AnxA2-GFP derivatives was determined by comparing the GFP signals with the immunofluorescence signals obtained by H21 antibody staining. Confocal microscopy used a LSM 510 Meta microscope (Carl Zeiss, Jena, Germany) equipped with a Plan/Apochromat 63× /1.4 oil immersion objective.
Statistical analysis
Graphs and associated statistics were generated using GraphPad Prism version 4.00 for Windows software (GraphPad Software, San Diego, CA). Mean values ± standard error of the mean were always calculated, and data were compared for significant differences using either the unpaired Student t test (in the case of 2 groups) or an analysis of variance (1-way ANOVA), for >2 groups. Once 1-way ANOVA identified a significant difference within several groups, comparison between 2 individual groups within that set was carried out using the Bonferroni test.
Results
AnxA2-S100A10 complex is involved in cAMP-induced VWF secretion
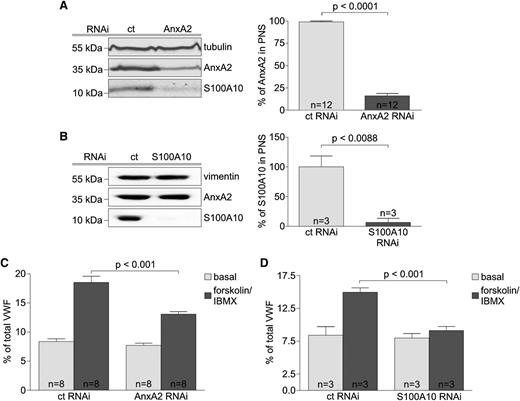
To determine whether AnxA2 and S100A10 play a functional role in endothelial WPB exocytosis evoked by cAMP-raising agents, we examined the effect of siRNA-mediated depletion of AnxA2 or S100A10 on forskolin-induced VWF release from HUVECs. Immunoblots of postnuclear supernatants (PNSs) from cells transiently transfected with AnxA2-specific, S100A10-specific, or nontargeting siRNAs revealed a marked depletion of AnxA2 (Figure 1A) and S100A10 (Figure 1B) in their respective transfected cells. As observed before,27 knockdown of AnxA2 leads to a concomitant reduction of S100A10 (Figure 1A) due to instability of the noncomplexed S100A10 protein. Down-regulation of AnxA2 (supplemental Figure 1) or S100A10 (data not shown) had no effect on the morphology and number of WPBs. However, VWF secretion in response to forskolin stimulation was significantly reduced in cells depleted of AnxA2 (Figure 1C) or S100A10 (Figure 1D).
Forskolin-evoked secretion of VWF is inhibited by knockdown of AnxA2 and S100A10. HUVECs were transiently transfected with either nontargeting siRNA duplexes (ct) or duplexes specific for AnxA2 or S100A10. (A-B) Forty-eight hours after transfection, PNSs were prepared and analyzed by immunoblotting for the presence of AnxA2 and S100A10. Probing for α-tubulin and vimentin served as loading controls. Signal intensities were quantified as described in the supplemental Methods, and results from different experiments are summarized in the right panels (n = number of independent experiments). Statistical significance was evaluated by unpaired Student t test. Bars represent mean ± standard error of the mean (SEM). (C-D) Quantification of VWF secretion in response to forskolin from HUVECs depleted of (C) AnxA2 or (D) S100A10. Forty-eight hours following transfection with siRNA duplexes, HUVECs were subjected to agonist treatment of 20 minutes. The amount of VWF released into the cell culture supernatant was then determined as described in Methods. Effects on secretion were quantified in ≥3 independent experiments, and 1-way ANOVA with Bonferroni test was performed to evaluate statistical significance. Bars represent mean ± SEM.
Forskolin-evoked secretion of VWF is inhibited by knockdown of AnxA2 and S100A10. HUVECs were transiently transfected with either nontargeting siRNA duplexes (ct) or duplexes specific for AnxA2 or S100A10. (A-B) Forty-eight hours after transfection, PNSs were prepared and analyzed by immunoblotting for the presence of AnxA2 and S100A10. Probing for α-tubulin and vimentin served as loading controls. Signal intensities were quantified as described in the supplemental Methods, and results from different experiments are summarized in the right panels (n = number of independent experiments). Statistical significance was evaluated by unpaired Student t test. Bars represent mean ± standard error of the mean (SEM). (C-D) Quantification of VWF secretion in response to forskolin from HUVECs depleted of (C) AnxA2 or (D) S100A10. Forty-eight hours following transfection with siRNA duplexes, HUVECs were subjected to agonist treatment of 20 minutes. The amount of VWF released into the cell culture supernatant was then determined as described in Methods. Effects on secretion were quantified in ≥3 independent experiments, and 1-way ANOVA with Bonferroni test was performed to evaluate statistical significance. Bars represent mean ± SEM.
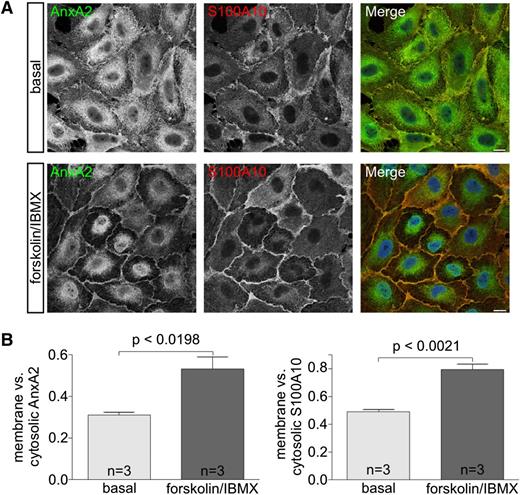
AnxA2 is a cytosolic protein that can also be found at the plasma membrane and the membrane of early endosomes following certain stimuli.18 In adrenal chromaffin cells, for example, nicotinic stimulation has been shown to trigger a translocation of cytosolic AnxA2 to the plasma membrane where it interacts with S100A10 to support catecholamine secretion.28 To determine whether a similar secretagogue-evoked translocation is observed in endothelial cells, we stimulated HUVECs with forskolin and recorded the distribution of AnxA2 and S100A10 before and after stimulation. Figure 2 reveals that both AnxA2 and S100A10 exhibit a more pronounced plasma membrane localization following forskolin treatment.
Subcellular localization of Anx A2 and S100A10. (A) HUVECs were incubated in basal medium or forskolin/3-Isobutyl-1-methylxanthine (IBMX)-containing stimulation medium for 20 minutes and subsequently fixed and permeabilized using 0.2% Triton-X100 in 4% paraformaldehyde (PFA)/phosphate-buffered saline. Cells were then stained with anti-AnxA2 (green) and anti-S100A10 (red) antibodies. 4,6 diamidino-2-phenylindole (DAPI) was used to label the nuclei (blue). Bars represent 10 µm. (B) For the quantification of the subcellular localization of AnxA2 and S100A10, signal intensities of both proteins in the cytoplasm and at the plasma membrane of 10 single cells per experiment were analyzed using ImageJ software. Ratios of membrane vs cytosolic intensities were calculated in 3 independent experiments, and the unpaired Student t test was performed to evaluate statistical significance. Bars represent mean ± SEM.
Subcellular localization of Anx A2 and S100A10. (A) HUVECs were incubated in basal medium or forskolin/3-Isobutyl-1-methylxanthine (IBMX)-containing stimulation medium for 20 minutes and subsequently fixed and permeabilized using 0.2% Triton-X100 in 4% paraformaldehyde (PFA)/phosphate-buffered saline. Cells were then stained with anti-AnxA2 (green) and anti-S100A10 (red) antibodies. 4,6 diamidino-2-phenylindole (DAPI) was used to label the nuclei (blue). Bars represent 10 µm. (B) For the quantification of the subcellular localization of AnxA2 and S100A10, signal intensities of both proteins in the cytoplasm and at the plasma membrane of 10 single cells per experiment were analyzed using ImageJ software. Ratios of membrane vs cytosolic intensities were calculated in 3 independent experiments, and the unpaired Student t test was performed to evaluate statistical significance. Bars represent mean ± SEM.
The above data suggest that AnxA2 and S100A10 translocate to the plasma membrane in forskolin-stimulated endothelial cells to support the secretion of VWF. However, because S100A10 is not stable in AnxA2-depleted cells, our above knockdown approaches do not allow a distinction between the involvement of the entire AnxA2-S100A10 complex or only S100A10 in the regulation of cAMP-mediated VWF secretion. To distinguish between these possibilities and to validate the specificity of our knockdown approaches, we carried out depletion/rescue experiments. HUVECs were depleted of AnxA2 (and thereby also S100A10) using siRNA, and we then determined whether cAMP-dependent VWF secretion can be restored using a siRNA-insensitive AnxA2 or a S100A10 construct, respectively. Figure 3A-C reveals that AnxA2 and S100A10 expression could be restored with the siRNA-resistant AnxA2 construct and that this reexpression also restored forskolin-induced secretion of VWF. Due to the limited stability of noncomplexed S100A10, the levels of S100A10 could not be elevated by transfection of a S100A10 expression construct into AnxA2-depleted cells (supplemental Figure 2A). Consequently, transfection of this S100A10 construct into HUVECs depleted of AnxA2 and thus also S100A10 could not restore the acute VWF release in response to forskolin (supplemental Figure 2B). To circumvent the problem of limited S100A10 stability in the absence of AnxA2 and to ultimately assess the potential role of noncomplexed S100A10 in cAMP-triggered VWF secretion, we generated a S100A10 mutant lacking the last 6 residues (YFP-S100A10-CΔ6). This mutant can still bind AnxA2 but has suffered a deletion of the major ubiquitinylation sites that are responsible for directing S100A10 to proteasomal degradation if these sites are not masked by AnxA2 binding.29,30 Figure 3D shows that this S100A10 mutant is indeed stably expressed in AnxA2-depleted HUVECs and thus most likely is resistant to ubiquitinylation. Moreover, YFP-S100A10-CΔ6 shows the same localization and forskolin-induced plasma membrane translocation as endogenous S100A10 in AnxA2-expressing HUVECs, indicating that it can form the heterotetrameric complex with AnxA2 (supplemental Figure 3). However, in the AnxA2-depleted cells that also suffer a loss of the endogenous S100A10, forskolin-induced VWF secretion cannot be restored by expression of the stable YFP-S100A10-CΔ6 mutant (Figure 3E). Together, these findings indicate that the entire AnxA2-S100A10 complex is required for mediating the acute release of VWF in response to cAMP-elevating agonists and that the individual subunits are not sufficient for this activity.
The entire AnxA2-S100A10 complex is involved in cAMP-dependent VWF release from HUVECs. HUVECs were transfected with siRNA specific for AnxA2 or nontargeting siRNA (ct RNAi). (A) Forty-eight hours later, cells were subjected to a second transfection using control siRNA, AnxA2 siRNA, or AnxA2 siRNA plus an siRNA-insensitive AnxA2 expression vector (AII-Rr). PNSs were prepared and analyzed by immunoblotting using rabbit polyclonal anti-AnxA2 antibodies for the detection of the total (endogenous and ectopically expressed) AnxA2 (total) and mouse monoclonal anti-AnxA2 antibodies (clone H28) for the specific detection of only ectopically expressed AnxA2 (ect.; see supplemental Methods). Probing with monoclonal α-tubulin antibodies was included as a loading control. (B) HUVECs transiently transfected with AnxA2 siRNA plus rescue plasmid (AII-Rr) were stained with (left) anti-AnxA2 antibodies specifically labeling the ectopically expressed AnxA2 (clone H28) and (right) anti-S100A10 antibodies directly coupled to Alexa Fluor 594. Nuclei were labeled with DAPI (blue). Scale bars represent 10 µm. Note the efficient restoration of AnxA2 and endogenous S100A10 levels in cells expressing the AnxA2 rescue construct (AII-Rr). (C) Cells transfected with siRNA (AnxA2 or nontargeting siRNA) or siRNA plus rescue plasmid (AII-Rr) were subjected to secretagogue-free and forskolin/IBMX-containing stimulation media for 20 min. Subsequently, the amount of released VWF was analyzed by enzyme-linked immunosorbent assay (ELISA). The effect on secretion was quantified in sets of 13 independent experiments, and 1-way ANOVA with Bonferroni test was performed to evaluate statistical significance. Bars represent mean ± SEM. (D) Forty-eight hours after the first siRNA transfection, HUVECs were subjected to a second round of transfection using either AnxA2 siRNA, AnxA2 siRNA plus a ubiquitinylation-resistant S100A10 mutant construct (YFP-S100A10-CΔ6), or control siRNA. Following another 48 h, PNS fractions were prepared and subjected to immunoblotting using rabbit polyclonal anti-AnxA2 and mouse monoclonal anti-S100A10 antibodies. Probing with monoclonal α-tubulin antibodies served as an internal loading control. Note the stability of the S100A10 mutant in cells depleted of AnxA2. (E) Transfections were carried out as described in D, and the amount of VWF released in response to forskolin was quantified by ELISA in sets of 3 independent experiments. One-way ANOVA with Bonferroni test was used for the evaluation of statistical significance. Bars represent mean ± SEM.
The entire AnxA2-S100A10 complex is involved in cAMP-dependent VWF release from HUVECs. HUVECs were transfected with siRNA specific for AnxA2 or nontargeting siRNA (ct RNAi). (A) Forty-eight hours later, cells were subjected to a second transfection using control siRNA, AnxA2 siRNA, or AnxA2 siRNA plus an siRNA-insensitive AnxA2 expression vector (AII-Rr). PNSs were prepared and analyzed by immunoblotting using rabbit polyclonal anti-AnxA2 antibodies for the detection of the total (endogenous and ectopically expressed) AnxA2 (total) and mouse monoclonal anti-AnxA2 antibodies (clone H28) for the specific detection of only ectopically expressed AnxA2 (ect.; see supplemental Methods). Probing with monoclonal α-tubulin antibodies was included as a loading control. (B) HUVECs transiently transfected with AnxA2 siRNA plus rescue plasmid (AII-Rr) were stained with (left) anti-AnxA2 antibodies specifically labeling the ectopically expressed AnxA2 (clone H28) and (right) anti-S100A10 antibodies directly coupled to Alexa Fluor 594. Nuclei were labeled with DAPI (blue). Scale bars represent 10 µm. Note the efficient restoration of AnxA2 and endogenous S100A10 levels in cells expressing the AnxA2 rescue construct (AII-Rr). (C) Cells transfected with siRNA (AnxA2 or nontargeting siRNA) or siRNA plus rescue plasmid (AII-Rr) were subjected to secretagogue-free and forskolin/IBMX-containing stimulation media for 20 min. Subsequently, the amount of released VWF was analyzed by enzyme-linked immunosorbent assay (ELISA). The effect on secretion was quantified in sets of 13 independent experiments, and 1-way ANOVA with Bonferroni test was performed to evaluate statistical significance. Bars represent mean ± SEM. (D) Forty-eight hours after the first siRNA transfection, HUVECs were subjected to a second round of transfection using either AnxA2 siRNA, AnxA2 siRNA plus a ubiquitinylation-resistant S100A10 mutant construct (YFP-S100A10-CΔ6), or control siRNA. Following another 48 h, PNS fractions were prepared and subjected to immunoblotting using rabbit polyclonal anti-AnxA2 and mouse monoclonal anti-S100A10 antibodies. Probing with monoclonal α-tubulin antibodies served as an internal loading control. Note the stability of the S100A10 mutant in cells depleted of AnxA2. (E) Transfections were carried out as described in D, and the amount of VWF released in response to forskolin was quantified by ELISA in sets of 3 independent experiments. One-way ANOVA with Bonferroni test was used for the evaluation of statistical significance. Bars represent mean ± SEM.
cAMP triggers dephosphorylation of AnxA2 via a calcineurin-like phosphatase
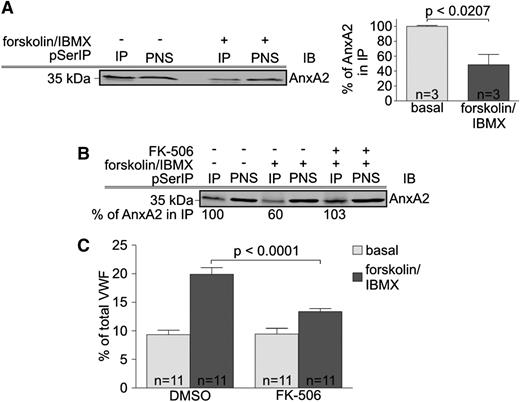
AnxA2 binds to S100A10 via its N-terminal domain,31 and phosphorylation at serine residues within this sequence can interfere with efficient complex formation, thereby representing a potential mode of regulating activities of the AnxA2-S100A10 complex.24,32 Therefore, we analyzed whether the serine phosphorylation status of AnxA2 is affected when endothelial VWF secretion is triggered by forskolin. Phosphoserine-containing proteins were isolated from HUVECs treated with either vehicle control or forskolin, and the resulting immunoprecipitates were probed for AnxA2. As shown in Figure 4A, the amount of Ser-phosphorylated AnxA2 was significantly reduced after forskolin stimulation.
Forskolin induces dephosphorylation of AnxA2. (A) HUVECs were incubated in forskolin/IBMX-containing stimulation medium or basal medium containing DMSO as vehicle control for 30 minutes, and PNSs were subjected to immunoprecipitation (IP) using anti–phosphoserine-coupled agarose. The amount of AnxA2 present in the PNS and the immunocomplexes was quantified by immunoblotting using mouse monoclonal anti-AnxA2 antibodies (clone HH7). One representative example is shown on the left. For quantification, AnxA2 levels in PNS fractions (input) were normalized to the respective level of the Ig heavy chain signals (internal loading control) and calculated as percentage of protein precipitated from unstimulated cells (right). Statistical significance of the results obtained from 3 independent experiments was evaluated by unpaired Student t test. (B) Serum-starved HUVECs were pretreated for 5 hours with 50 nM FK-506 or DMSO as vehicle control at 37°C. Subsequently, cells were incubated in forskolin/IBMX-containing stimulation or basal medium for 30 minutes, and PNSs were subjected to anti–phospho-serine IP as described in A. PNS and IP fractions were probed for AnxA2, and a representative immunoblot of 2 independent experiments is shown. Numbers below the blot indicate AnxA2 levels in the IPs that were calculated as percent of protein precipitated from unstimulated cells as described in A. (C) HUVECs were preincubated in medium containing FK-506 or DMSO (vehicle control) and then subjected to forskolin/IBMX treatment as outlined in Methods. The amount of VWF released into the cell culture supernatant was analyzed by ELISA, and statistical significance of the results obtained from 11 independent experiments was evaluated by unpaired Student t test. Bars represent mean ± SEM. The basal secretion is not affected by FK-506 treatment, whereas the calcineurin inhibitor significantly reduces the forskolin-induced secretion.
Forskolin induces dephosphorylation of AnxA2. (A) HUVECs were incubated in forskolin/IBMX-containing stimulation medium or basal medium containing DMSO as vehicle control for 30 minutes, and PNSs were subjected to immunoprecipitation (IP) using anti–phosphoserine-coupled agarose. The amount of AnxA2 present in the PNS and the immunocomplexes was quantified by immunoblotting using mouse monoclonal anti-AnxA2 antibodies (clone HH7). One representative example is shown on the left. For quantification, AnxA2 levels in PNS fractions (input) were normalized to the respective level of the Ig heavy chain signals (internal loading control) and calculated as percentage of protein precipitated from unstimulated cells (right). Statistical significance of the results obtained from 3 independent experiments was evaluated by unpaired Student t test. (B) Serum-starved HUVECs were pretreated for 5 hours with 50 nM FK-506 or DMSO as vehicle control at 37°C. Subsequently, cells were incubated in forskolin/IBMX-containing stimulation or basal medium for 30 minutes, and PNSs were subjected to anti–phospho-serine IP as described in A. PNS and IP fractions were probed for AnxA2, and a representative immunoblot of 2 independent experiments is shown. Numbers below the blot indicate AnxA2 levels in the IPs that were calculated as percent of protein precipitated from unstimulated cells as described in A. (C) HUVECs were preincubated in medium containing FK-506 or DMSO (vehicle control) and then subjected to forskolin/IBMX treatment as outlined in Methods. The amount of VWF released into the cell culture supernatant was analyzed by ELISA, and statistical significance of the results obtained from 11 independent experiments was evaluated by unpaired Student t test. Bars represent mean ± SEM. The basal secretion is not affected by FK-506 treatment, whereas the calcineurin inhibitor significantly reduces the forskolin-induced secretion.
Whereas PKC has been shown to catalyze Ser phosphorylation of AnxA2 in endothelial cells, thereby destabilizing the AnxA2-S100A10 complex,24 Borthwick et al33 have shown that a cAMP/PKA-activated calcineurin-like phosphatase is involved in dephosphorylating AnxA2 in airway and intestinal epithelial cells, resulting in a stabilization of the complex with S100A10. Therefore, we examined whether such pathway is also triggered in forskolin-treated HUVECs. In line with previous observations revealing a role of PKA in cAMP-evoked WPB exocytosis,13 we first showed that treatment of HUVECs with the PKA inhibitor H89 interfered with forskolin-triggered VWF secretion, indicating that PKA is an intermediate in this secretory response (supplemental Figure 4). Next, we analyzed whether the cAMP-mediated PKA activation in HUVECs triggers a calcineurin-like phosphatase that could dephosphorylate AnxA2. Therefore, HUVECs were pretreated with FK-506, a specific calcineurin inhibitor, and then stimulated with forskolin, followed by immunoprecipitation of total pSer proteins. Probing of these immunoprecipitates for AnxA2 revealed that calcineurin inhibition increased the amount of Ser-phosphorylated AnxA2 in the forskolin-treated cells to a level similar to that of unstimulated HUVECs, indicating that calcineurin dephosphorylates AnxA2 in response to forskolin stimulation (Figure 4B). We then elucidated whether calcineurin activity is also required for VWF secretion by assessing the effect of FK-506 treatment on the cAMP-triggered release of VWF. First, we established that FK-506 treatment alone does not trigger VWF secretion in resting HUVECs (supplemental Figure 5). This finding is in contrast to a previous report showing a FK-506–induced increase in WPB exocytosis in the absence of other stimuli.34 The reason for these differing results is not known but could be due to the different HUVEC culture conditions because we cultivate our HUVECs in endothelial growth medium, whereas Nolasco et al34 used normal M199 plus fetal calf serum. Although FK-506 did not affect basal VWF secretion (supplemental Figure 5), the inhibitor markedly reduced the forskolin-triggered secretion of VWF (Figure 4C). Thus, our findings suggest that a calcineurin-like phosphatase is a positive regulator of cAMP-induced WPB exocytosis and that this phosphatase exerts its effect by dephosphorylation of AnxA2.
Ser-11 phosphorylation of AnxA2 interferes with cAMP-dependent VWF secretion
To identify the residue in AnxA2 that is dephosphorylated following cAMP stimulation of endothelial cells and thus regulates AnxA2-S100A10 complex formation in the course of cAMP-dependent VWF secretion, we concentrated on Ser-11. Although other potential serine phosphorylation sites exist in the N-terminal domain of AnxA2,32 Ser-11 is the most likely candidate for several reasons. First, Ser-11 lies within the S100A10 binding sequence of AnxA2 (residues 1-14),31 and the high-resolution crystal structure of an N-terminal AnxA2 peptide complexed with S100A10 has mapped Ser-11 to the actual binding interface.35 Second, a phosphomimicking mutation at this site, S11D, markedly reduces the affinity of AnxA2 and the N-terminal AnxA2 peptide for S100A10 in in vitro binding experiments.36,37 To analyze whether this mutation also interferes with AnxA2-S100A10 complex formation in cells, we developed a new model system to probe for intracellular AnxA2-S100A10 interactions. This system is based on a chimeric protein consisting of the N-terminal 18 residues of AnxA2 fused to a full-length S100A10 chain. The mutant, which we termed XM-AnxA2, has a bivalent character because the S100A10 part can dimerize or bind an endogenous S100A10 molecule and the AnxA2 part can also bind to S100A10. When expressed in cells, this leads to the formation of larger aggregates that recruit endogenous AnxA2 due to its S100A10 binding capacity.26 Ectopically expressed AnxA2 derivatives capable of binding S100A10 should also be recruited to the aggregates, providing a direct means of analyzing their complex formation capacity. To avoid potential long-term toxic effects of XM-AnxA2, the mutant was expressed in a tetracycline-regulated manner in MDCK cells that were chosen because the formation of XM-induced AnxA2/S100A10 aggregates is particularly pronounced in this cell type.26 Cells stably expressing XM-AnxA2 were transiently transfected with WT AnxA2 or 2 Ser-11 AnxA2 mutants, S11D and S11A, and the coclustering of the these AnxA2 derivatives with XM-AnxA2, which can only occur following an interaction with the S100A10 part, was analyzed by immunofluorescence. Figure 5 reveals the XM-AnxA2 induced clusters and also shows that ectopically expressed WT AnxA2 was recruited to these clusters (Figure 5B). S11A-AnxA2 was also found in the clusters (Figure 5C), whereas S11D-AnxA2 showed a general cytosolic distribution with no significant enrichment at the sites of XM-AnxA2 aggregates (Figure 5D). This was also evident when we determined colocalization coefficients of the two AnxA2 mutants with the XM-AnxA2 clusters (Figure 5F).
Phosphorylation of Ser-11 of AnxA2 interferes with S100A10 complex formation in MDCK cells. Subcellular localization of different AnxA2 mutants in MDCK cells stably transfected with the tetracycline-regulated XM-AnxA2 construct that induces AnxA2/S100A10 aggregates. MDCK-tTA-XM cells were cultivated either in (A) tetracycline-containing medium or in (B-E) the absence of tetracycline to induce expression of the XM construct. Subsequently, cells were transfected with the respective GFP-tagged AnxA2 constructs ([A-B] WT, [C] S11A, [D] S11D) or (E) a GFP expression vector as control. Twenty-four hours after transfection, cells were fixed, permeabilized, and stained with anti-S100A10 antibodies (clone H21) that recognize the XM derivative. WT- and S11A-AnxA2 but not S11D-AnxA2 are recruited to these clusters. Scale bars represent 10 µm. (F) Colocalization coefficients of S100A10-stained XM clusters and the respective AnxA2-GFP constructs of 7 single cells per experiment were determined using BioImageXD software, and statistical significance of 3 independent experiments was analyzed by 1-way ANOVA with Bonferroni test. Bars represent mean ± SEM.
Phosphorylation of Ser-11 of AnxA2 interferes with S100A10 complex formation in MDCK cells. Subcellular localization of different AnxA2 mutants in MDCK cells stably transfected with the tetracycline-regulated XM-AnxA2 construct that induces AnxA2/S100A10 aggregates. MDCK-tTA-XM cells were cultivated either in (A) tetracycline-containing medium or in (B-E) the absence of tetracycline to induce expression of the XM construct. Subsequently, cells were transfected with the respective GFP-tagged AnxA2 constructs ([A-B] WT, [C] S11A, [D] S11D) or (E) a GFP expression vector as control. Twenty-four hours after transfection, cells were fixed, permeabilized, and stained with anti-S100A10 antibodies (clone H21) that recognize the XM derivative. WT- and S11A-AnxA2 but not S11D-AnxA2 are recruited to these clusters. Scale bars represent 10 µm. (F) Colocalization coefficients of S100A10-stained XM clusters and the respective AnxA2-GFP constructs of 7 single cells per experiment were determined using BioImageXD software, and statistical significance of 3 independent experiments was analyzed by 1-way ANOVA with Bonferroni test. Bars represent mean ± SEM.
We also investigated the complex formation of WT AnxA2 and the Ser-11 mutants with S100A10 in HUVECs by performing coimmunoprecipitation experiments. HUVECs were transiently transfected with the different AnxA2 constructs, and the ectopically (ect.) expressed AnxA2 was specifically precipitated using a monoclonal antibody (H28) that does not react with the endogenous AnxA2 (see supplemental Methods for details). The resulting immunoprecipitates were analyzed by immunoblotting for ectopically expressed AnxA2 and endogenous S100A10. As shown in Figure 6, the amount of coprecipitated S100A10 in cells expressing AnxA2-S11A was increased compared with cells expressing WT-AnxA2. In contrast, the interaction between AnxA2-S11D and S100A10 was markedly reduced, albeit not completely abolished, indicative of a reduced binding affinity. Thus, the phosphomimicking S11D mutation in AnxA2 prevents efficient interaction with S100A10 and can therefore be used as a tool to study the importance of phosphorylation at this site in cAMP-dependent VWF secretion.
The phosphomimicking S11D mutation in AnxA2 attenuates the interaction between AnxA2 and S100A10 in HUVECs. HUVECs were transiently transfected with WT-, S11A-, or S11D-AnxA2, respectively. Forty-eight hours after transfection, PNSs were prepared and subjected to immunoprecipitation using mouse monoclonal antibodies only recognizing the ectopically expressed AnxA2 derivatives (clone H28; supplemental Methods). The amount of AnxA2 derivative (ect. AnxA2) and S100A10 present in the PNSs and the immunoprecipitates was quantified as described in supplemental Methods. For quantification, AnxA2 levels in IP fractions were normalized to the respective level in the PNS fraction (input), and the amount of coprecipitated S100A10 was calculated as percentage of precipitated AnxA2 (bar diagram). Statistical significance of the results obtained from 3 independent experiments was evaluated by 1-way ANOVA with the unpaired Student t test. Bars represent mean ± SEM.
The phosphomimicking S11D mutation in AnxA2 attenuates the interaction between AnxA2 and S100A10 in HUVECs. HUVECs were transiently transfected with WT-, S11A-, or S11D-AnxA2, respectively. Forty-eight hours after transfection, PNSs were prepared and subjected to immunoprecipitation using mouse monoclonal antibodies only recognizing the ectopically expressed AnxA2 derivatives (clone H28; supplemental Methods). The amount of AnxA2 derivative (ect. AnxA2) and S100A10 present in the PNSs and the immunoprecipitates was quantified as described in supplemental Methods. For quantification, AnxA2 levels in IP fractions were normalized to the respective level in the PNS fraction (input), and the amount of coprecipitated S100A10 was calculated as percentage of precipitated AnxA2 (bar diagram). Statistical significance of the results obtained from 3 independent experiments was evaluated by 1-way ANOVA with the unpaired Student t test. Bars represent mean ± SEM.
To analyze the potential regulatory importance of AnxA2 Ser-11 phosphorylation in cAMP-triggered VWF secretion, we next generated siRNA-insensitive versions of S11A-AnxA2 and S11D-AnxA2 and used these in rescue experiments with HUVECs depleted of AnxA2. Western blot analyses show that both AnxA2 mutants were expressed to similar levels in cells depleted of endogenous AnxA2 (Figure 7A-B). As revealed by corresponding immunofluorescence studies, S11A- but not S11D-AnxA2 also restored the stable expression of endogenous S100A10, most likely due to more efficient complex formation (Figure 7C-D). In line with a crucial function of the complex, cAMP-mediated VWF secretion was only efficiently restored on ectopic expression of the S11A-AnxA2 mutant that is capable of high-affinity S100A10 binding (Figure 7E). In contrast, reexpression of the S11D-AnxA2 mutant only resulted in a minor rescue that is most likely due to some residual complex formation observed for this mutant in the coimmunoprecipitation assay (Figure 7F). Together these results indicate that introduction of a negative, phosphomimicking charge at Ser-11 of AnxA2 attenuates forskolin-induced secretion of VWF by preventing efficient AnxA2-S100A10 complex formation.
AnxA2 containing a phosphomimicking mutation at Ser-11 does not promote forskolin-induced VWF secretion. Forty-eight hours after transfection with AnxA2 siRNA or nontargeting siRNA (ct RNAi), HUVECs were transfected a second time either with siRNA specific for AnxA2, control siRNA, or with AnxA2 siRNA together with siRNA-insensitive AnxA2 mutant constructs (S11A or S11D). Rescue of AnxA2 protein levels following expression of the (A) AII-Rr-S11A or (B) AII-Rr-S11D constructs was verified by immunoblotting using polyclonal rabbit anti-AnxA2 antibodies for the detection of endogenous and exogenous AnxA2 (total) or monoclonal mouse anti-AnxA2 antibodies (clone H28) for the detection of only ectopically expressed S11A- and S11D-AnxA2 (ect.). Probing with anti–α-tubulin antibodies was used as internal loading control. (C-D) HUVECs transiently transfected with AnxA2 siRNA plus the respective rescue plasmids ([C] AII-Rr-S11A and [D] AII-Rr-S11D) were stained with (left) anti-AnxA2 antibodies (clone H28 to label ectopically expressed AnxA2) and (right) anti-S100A10 antibodies directly coupled to Alexa Fluor 594. Nuclei were labeled with DAPI (blue). Note the restoration of endogenous S100A10 levels in cells ectopically expressing AII-Rr-S11A but not in cells expressing AII-Rr-S11D. Scale bars represent 10 µm. (E-F) HUVECs transfected with siRNA and the respective rescue constructs were subjected to forskolin/IBMX treatment, and VWF secretion was analyzed by ELISA. The effect on secretion was quantified in (C) 9 and (D) 11 independent experiments. One-way ANOVA with Bonferroni test was used for the quantification of statistical significance. Bars represent mean ± SEM.
AnxA2 containing a phosphomimicking mutation at Ser-11 does not promote forskolin-induced VWF secretion. Forty-eight hours after transfection with AnxA2 siRNA or nontargeting siRNA (ct RNAi), HUVECs were transfected a second time either with siRNA specific for AnxA2, control siRNA, or with AnxA2 siRNA together with siRNA-insensitive AnxA2 mutant constructs (S11A or S11D). Rescue of AnxA2 protein levels following expression of the (A) AII-Rr-S11A or (B) AII-Rr-S11D constructs was verified by immunoblotting using polyclonal rabbit anti-AnxA2 antibodies for the detection of endogenous and exogenous AnxA2 (total) or monoclonal mouse anti-AnxA2 antibodies (clone H28) for the detection of only ectopically expressed S11A- and S11D-AnxA2 (ect.). Probing with anti–α-tubulin antibodies was used as internal loading control. (C-D) HUVECs transiently transfected with AnxA2 siRNA plus the respective rescue plasmids ([C] AII-Rr-S11A and [D] AII-Rr-S11D) were stained with (left) anti-AnxA2 antibodies (clone H28 to label ectopically expressed AnxA2) and (right) anti-S100A10 antibodies directly coupled to Alexa Fluor 594. Nuclei were labeled with DAPI (blue). Note the restoration of endogenous S100A10 levels in cells ectopically expressing AII-Rr-S11A but not in cells expressing AII-Rr-S11D. Scale bars represent 10 µm. (E-F) HUVECs transfected with siRNA and the respective rescue constructs were subjected to forskolin/IBMX treatment, and VWF secretion was analyzed by ELISA. The effect on secretion was quantified in (C) 9 and (D) 11 independent experiments. One-way ANOVA with Bonferroni test was used for the quantification of statistical significance. Bars represent mean ± SEM.
Discussion
The regulated secretion of VWF from endothelial cells represents a critical response to vascular injury and local inflammation. It can be triggered by several agonists, most of them acting through either Ca2+ or cAMP as the relevant second messengers. Although elevation of both Ca2+ and cAMP levels induces exocytosis of the VWF storage organelles, the WPBs, several differences exist between the 2 pathways. For example, Ca2+-raising agonists such as histamine or thrombin trigger a rapid exocytotic response that can lead to the emptying of a majority of the intracellular WPBs, whereas epinephrine and vasopressin that increase cAMP levels induce a slower VWF release. The latter only involves a fraction of the intracellular WPBs and also leads to a perinuclear clustering of many of the remaining WPBs.38 These differential responses could be of physiological relevance as Ca2+-elevating secretagogues often act to rapidly elicit local responses to vascular injury, whereas cAMP-increasing agonists function more systemically, thereby controlling general VWF levels in the vasculature.11,39 Whereas some molecular aspects of the intracellular signaling pathway downstream of Ca2+ elevation have been described, much less is known about components of the cAMP-triggered exocytosis machinery, and it is also not clear whether and when both pathways (Ca2+ and cAMP) converge to finally trigger the exocytosis of WPBs.
Here we show that the AnxA2-S100A10 complex is a new component of the cAMP-dependent pathway of VWF release, and more importantly, we identify for the first time a phosphorylation/dephosphorylation target activated in the course of cAMP-triggered VWF secretion. Although evidence for a role of PKA in cAMP-dependent VWF secretion has been obtained through inhibitor experiments,16,17,40,41 PKA substrates in this pathway are not known. Although counterintuitive at a first glance, we show that PKA (indirectly) triggers a dephosphorylation of AnxA2, thereby promoting AnxA2-S100A10 complex formation that in turn is required for efficient WPB exocytosis. As initially identified in epithelial cells,33 this dephosphorylation is mediated by a calcineurin-like phosphatase that is activated by PKA. Thus, in line with previous observations, our results support the following order of events. Endothelial stimulation by cAMP-elevating agonists results in PKA activation that triggers a calcineurin-like phosphatase dephosphorylating AnxA2. Dephosphorylated AnxA2 can bind S100A10 and thereby form the heterotetrameric AnxA2-S100A10 complex that is required for WPB exocytosis. Thereby, a phosphorylation/dephosphorylation switch in AnxA2 is exploited as an elegant means of controlling WPB exocytosis. Future experiments are needed to reveal whether this pathway also operates in the recently described PKA-dependent release of VWF that is triggered by Shiga toxin 2B and occurs without elevation of intracellular cAMP levels.17
Previous in vitro approaches identified PKC phosphorylation sites in the N-terminal domain of AnxA2 that affect the stability of the AnxA2-S100A10 complex,32 and a recent study revealed a PKC-dependent serine phosphorylation of AnxA2 in endothelial cells that induced a dissociation from S100A10.24 Thus, PKC seems to be primarily responsible for complex-destabilizing serine phosphorylation in AnxA2. In contrast, PKA through activation of a calcineurin-like phosphatase stabilizes AnxA2-S100A10 complexes by dephosphorylation of AnxA2 as shown in airway epithelial cells33 and now in HUVECs in the course of forskolin-induced VWF secretion. The N-terminal domain of AnxA2 contains ≥3 putative PKC phosphorylation sites at residues Ser-11, Ser-21, and Ser-25, only one of which (Ser-11) is located in the N-terminal amphiphatic α-helix (residues 1-14) that interacts with S100A10.31 Previous in vitro binding studies using an AnxA2 mutant peptide with a phosphomimicking replacement at that site revealed a significantly reduced affinity for S100A10.36 Furthermore, the crystal structure of a complex consisting of S100A10 and the N-terminal AnxA2 peptide shows that Ser-11 lies in the binding pocket and that a phosphorylation or negatively charged side chain at this site could probably not be accommodated in the pocket.35 Thus, all in vitro findings point to Ser-11 as the crucial phosphorylation site involved in regulating AnxA2-S100A10 complex stability. Our mutant experiments now provide evidence for the regulatory importance of this site in cells. We show that complex formation between AnxA2 and S100A10 is required for cAMP-induced VWF release and that in AnxA2-depleted endothelial cells, this function can be restored efficiently by reexpression of WT- and S11A- but not S11D-AnxA2. Introduction of a phosphomimicking charge at position 11 therefore prevents complex formation with S100A10, and the remaining monomeric AnxA2 cannot substitute for the AnxA2-S100A10 complex in mediating the cAMP-induced exocytosis of WPBs.
The exact site of action of the AnxA2-S100A10 complex in regulated WPB exocytosis is not known. AnxA2-S100A10 is not found on WPBs (Figure 2),20 and our experiments show that the complex translocates from the cytosol to the plasma membrane following endothelial stimulation with cAMP-elevating agents. This corresponds to some extent to the situation in adrenal chromaffin cells that show a cytosol to plasma membrane translocation of AnxA2 in response to nicotinic stimulation.28 In chromaffin cells, this translocation appears to promote the formation of cholesterol-rich lipid microdomains required for efficient dense core granule exocytosis.21 A similar scenario might hold true in HUVECs in the course of WPB exocytosis as endothelial AnxA2-S100A10 localizes to the plasma membrane following forskolin stimulation and as membrane-bound AnxA2-S100A10 can be liberated with cholesterol sequestering agents.42 Because only the AnxA2-S100A10 complex but not monomeric AnxA2 is thought to efficiently promote microdomain formation at very low Ca2+ levels,18 regulation of complex stability by phosphorylation/dephosphorylation at Ser-11 of AnxA2 could provide a unique and specific way of controlling VWF secretion in the absence of significant Ca2+ elevation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ursula Rescher, Nina Quiskamp, Tarek Chehab, Tim Stobernack, and Annette Janning (University of Muenster) for providing mutant constructs, performing some of the PKA inhibitor experiments, and helpful discussions. Claude Klee (National Institutes of Health) provided important advice on the calcineurin inhibitor experiments.
This work was supported by Deutsche Forschungsgemeinschaft grants Ge 514/6-1, SFB 629/A1, and EXC 1003.
Authorship
Contribution: I.B. and J.D. performed research, analyzed data, and wrote parts of the manuscript; D.Z. performed research and analyzed data; and V.G. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Volker Gerke, Institute of Medical Biochemistry, Centre for Molecular Biology of Inflammation, University of Münster, Von-Esmarch-Str. 56, 48149 Münster, Germany; e-mail: gerke@uni-muenster.de.


![Figure 5. Phosphorylation of Ser-11 of AnxA2 interferes with S100A10 complex formation in MDCK cells. Subcellular localization of different AnxA2 mutants in MDCK cells stably transfected with the tetracycline-regulated XM-AnxA2 construct that induces AnxA2/S100A10 aggregates. MDCK-tTA-XM cells were cultivated either in (A) tetracycline-containing medium or in (B-E) the absence of tetracycline to induce expression of the XM construct. Subsequently, cells were transfected with the respective GFP-tagged AnxA2 constructs ([A-B] WT, [C] S11A, [D] S11D) or (E) a GFP expression vector as control. Twenty-four hours after transfection, cells were fixed, permeabilized, and stained with anti-S100A10 antibodies (clone H21) that recognize the XM derivative. WT- and S11A-AnxA2 but not S11D-AnxA2 are recruited to these clusters. Scale bars represent 10 µm. (F) Colocalization coefficients of S100A10-stained XM clusters and the respective AnxA2-GFP constructs of 7 single cells per experiment were determined using BioImageXD software, and statistical significance of 3 independent experiments was analyzed by 1-way ANOVA with Bonferroni test. Bars represent mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/6/10.1182_blood-2012-12-475251/4/m_1042f5.jpeg?Expires=1765002329&Signature=VLMsgpyACNjz32cOTbezqxErqW22XUdfy-K2X1Uh~0cdA1u1a8Lj1JDUnCrlmDUWo7rNXrb~7wZ7ljhSiLaSwpuFHuqaOaGjVR9U7zPmbLFPtKXnfQhPUbW-e6TZEyCp9cawFeaKc9F7gLMUxCTkLM~J4OSfF1vGiT-HzsqYh4ky91RUW3Lh1ts5Ar6P2Nu42fhOJ~CWE3aJSF-kqpgpz0dykEOBlFlJaFtnU5odL-Oej9I-qkmIIkH-SPu17m8~BV2Fc6AGnDC-Y442jVWH66msYgX5lfW797ypRrA3iJjU9kXo-DLIMRA0072e7kzdJpqdnoO10oEH9A4~721M1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. AnxA2 containing a phosphomimicking mutation at Ser-11 does not promote forskolin-induced VWF secretion. Forty-eight hours after transfection with AnxA2 siRNA or nontargeting siRNA (ct RNAi), HUVECs were transfected a second time either with siRNA specific for AnxA2, control siRNA, or with AnxA2 siRNA together with siRNA-insensitive AnxA2 mutant constructs (S11A or S11D). Rescue of AnxA2 protein levels following expression of the (A) AII-Rr-S11A or (B) AII-Rr-S11D constructs was verified by immunoblotting using polyclonal rabbit anti-AnxA2 antibodies for the detection of endogenous and exogenous AnxA2 (total) or monoclonal mouse anti-AnxA2 antibodies (clone H28) for the detection of only ectopically expressed S11A- and S11D-AnxA2 (ect.). Probing with anti–α-tubulin antibodies was used as internal loading control. (C-D) HUVECs transiently transfected with AnxA2 siRNA plus the respective rescue plasmids ([C] AII-Rr-S11A and [D] AII-Rr-S11D) were stained with (left) anti-AnxA2 antibodies (clone H28 to label ectopically expressed AnxA2) and (right) anti-S100A10 antibodies directly coupled to Alexa Fluor 594. Nuclei were labeled with DAPI (blue). Note the restoration of endogenous S100A10 levels in cells ectopically expressing AII-Rr-S11A but not in cells expressing AII-Rr-S11D. Scale bars represent 10 µm. (E-F) HUVECs transfected with siRNA and the respective rescue constructs were subjected to forskolin/IBMX treatment, and VWF secretion was analyzed by ELISA. The effect on secretion was quantified in (C) 9 and (D) 11 independent experiments. One-way ANOVA with Bonferroni test was used for the quantification of statistical significance. Bars represent mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/6/10.1182_blood-2012-12-475251/4/m_1042f7.jpeg?Expires=1765002329&Signature=DzsupGBGQ2FyLNrEQsmMg2RtBgl5bKA~Rr0FjT-u2hZHMbf-S9Eh1bDwk4FQ6cF~eRDHACgEYQAFv-KIbRFiJekVjzcVwUjkqzrm3z5AnGn5VahNBe5dLiTxpYkAYs~Kebgx~TWyBmf8OMM-qvfM9R7bzE40lLXY4F4ZaCkoEI~yDaTaByikq0K4h-eOnDSqEMK492jTsmVBOSTk8bNq1GUNk~1wuQ5CCgvSinrqQNGjADPS6kEnM7LHvTJ3tqlHaa5MAGFd~4EfEo1gasoD8C5Y3KP~MYIDifkgoV8IGx4jVgjht682zPx7gQHnUpVaCP3tTwXhBTTOHSLQ3HLLeA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)