Key Points
Sphk2 provides a source of intracellular S1P that tightly controls thrombopoiesis by regulating SFK expression and activity in MKs.
Modulation of intracellular S1P by regulating Sphk2 may provide a new strategy to enhance platelet production in patients with thrombocytopenia.
Abstract
Human megakaryocytes (MKs) release trillions of platelets each day into the circulation to maintain normal homeostatic platelet levels. We have previously shown that extracellular sphingosine 1-phosphate (S1P) plays a key role in thrombopoiesis via its receptor S1pr1. In addition to its role as an extracellular mediator, S1P can also function as a second messenger in the intracellular compartment. Although signaling via intracellular S1P is involved in various cellular processes, a role in thrombopoiesis has not been examined. Sphingosine kinases are the key enzymes that produce intracellular S1P. Here we report that sphingosine kinase 2 (Sphk2) is the major messenger RNA species present in MKs. Sphk2 predominantly localizes to the nucleus and is the major source of intracellular S1P in MKs. Loss of Sphk2 significantly reduced intracellular S1P in MKs and downregulated the expression and activity of Src family kinases (SFKs). Loss of Sphk2 and inhibition of SFK activity resulted in defective intravascular proplatelet shedding, the final stage of thrombopoiesis. Correspondingly, mice lacking Sphk2 in the hematopoietic system display thrombocytopenia. Together, our data suggest that Sphk2 provides the source of intracellular S1P that controls thrombopoiesis, which is associated with SFK expression and activity in MKs.
Introduction
The presence of sufficient numbers of functional platelets in the blood is critical for proper hemostasis. Platelets (150 × 109/L to 400 × 109/L) circulate in normal human blood with a short life span (5-9 days).1 To maintain a normal number of platelets in the circulation, it is estimated that the platelet precursor cells, megakaryocytes (MKs), release 15 × 109 to 40 × 109 platelets each day. However, MKs are an extremely rare cell population in bone marrow (BM) (<0.01% of BM cells), indicating that each MK has to produce 1000 to 3000 platelets during its lifetime.2 To efficiently release platelets into the circulation, MKs strategically locate close to BM sinusoids and protrude long cytoplasmic extensions through the endothelium into the sinusoidal lumen.3 The long cytoplasmic processes extending from MKs are termed proplatelets (PPs).4 PPs consist of multiple platelet-sized beads connected by cytoplasmic bridges. Previous investigations have shown that platelets are released from the tips of PPs4 and that microtubule and cortical forces regulate terminal platelet size.5 However, little is known about the molecular mechanisms that control the release of platelets from MKs.
Sphingosine 1-phosphate (S1P) is an important bioactive lipid with pleiotropic cellular functions.6 We have shown recently that extracellular S1P serves as a critical directional cue guiding the elongation of megakaryocytic PP extensions from the interstitium into BM sinusoids and triggering the subsequent shedding of PPs into the blood acting via its receptor S1pr1.7 Emerging evidence suggests that in addition to extracellular S1P, intracellular S1P acting as a second messenger also critically controls various cellular functions.8 The biosynthesis of S1P is catalyzed by sphingosine kinases (Sphks),9 which are widely expressed across different species from plants to humans. Two Sphk isoenzymes have been identified in mammals, Sphk1 and Sphk2. Although MKs have been reported to produce S1P, the relevance of distinct Sphk isoenzymes for intracellular S1P generation and their role in physiological thrombopoiesis remain unclear.
Here we report that Sphk2 messenger RNA (mRNA) is the major Sphk transcript expressed in MKs. Sphk2 produced intracellular S1P and predominantly localized in the nucleus of MKs to regulate Src family kinase (SFK) gene expression. Loss of Sphk2 reduced intracellular S1P synthesis and downregulated SFK expression and activity. We further show that SFK activity controls the shedding of PPs from MKs into the blood. Using multiphoton intravital microscopy (MP-IVM), we show that Sphk2−/− mice have a defect in PP shedding inside BM sinusoidal lumens that can be phenocopied by pharmacologically blocking SFK activity. This defect could explain our finding that mice lacking Sphk2 in the hematopoietic system display thrombocytopenia. Taken together, our data point to a role of Sphk2 and intracellular S1P in platelet biogenesis, which is related to the expression and activity of SFKs.
Methods
All experiments performed on animals were approved by the Bavarian government. Mice, quantitative reverse transcription–polymerase chain reaction, blood cell and serum Tpo measurements, immunostaining, MK cultivation, MK colony-forming unit (CFU-MK) assay, flow cytometric analysis, western blot analysis, 3H-S1P measurement, 2-photon intravital imaging of the BM, live cell imaging, and statistics are described in detail in the supplemental Materials and methods (see the Blood Web site).
Results
Sphk2 is required for proper platelet homeostasis
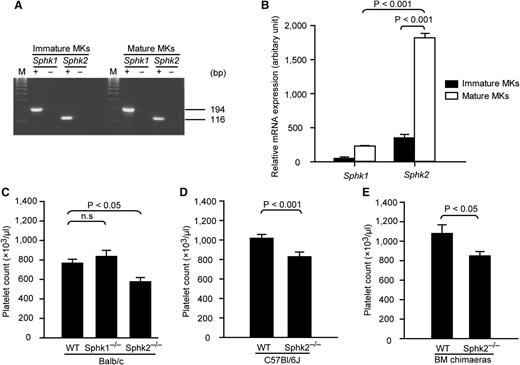
To investigate a possible role of endogenously synthesized S1P in megakaryopoiesis and thrombopoiesis, we first determined whether MKs express either of the Sphk isoforms, Sphk1 and Sphk2. Our results showed that both Sphk1 and Sphk2 are expressed in MKs (Figure 1A; for primers, see supplemental Table 1). Moreover, Sphk2 was significantly upregulated during megakaryocytic maturation and was the major Sphk mRNA species in mature MKs (Figure 1B). Next, to determine whether Sphk’s are involved in the regulation of megakaryopoiesis or thrombopoiesis, we measured the peripheral platelet counts in mice lacking Sphk1 or Sphk2. Sphk1−/− mice had a normal number of peripheral platelets, whereas Sphk2−/− mice displayed reduced platelet counts (Figure 1C). A similar thrombocytopenia was found in Sphk2−/− mice from either a Balb/c or C57Bl/6J background (Figure 1C-D; supplemental Table 2), suggesting that this phenotype is independent of the genetic background. Red blood cells, white blood cells, and lymphocyte counts are unchanged in Sphk2−/− mice, implying that loss of Sphk2 has no effect on the differentiation of other hematopoietic lineages than the megakaryocytic lineage (supplemental Table 2). To evaluate the influence of Sphk2 from the endothelium or other nonhematopoietic sources on thrombopoiesis, we generated BM chimeras by transferring BM cells from wild-type (WT) or Sphk2−/− mice into lethally irradiated WT recipient mice. Sphk2−/− BM cells successfully reconstituted the hematopoietic system in lethally irritated WT recipient mice (supplemental Table 3), suggesting normal repopulation functions of Sphk2−/− hematopoietic stem and progenitor cells. However, Sphk2−/− BM chimeras developed thrombocytopenia (Figure 1E; supplemental Table 3). This indicated that loss of Sphk2 in the hematopoietic system was causative for thrombocytopenia. Collectively, these results demonstrate that Sphk1 is dispensable, but Sphk2 is required for megakaryopoiesis or thrombopoiesis under physiological conditions. Therefore, we focused on the function of Sphk2 in thrombopoiesis in the subsequent investigations.
MKs express Sphk2 and Sphk2-deficient mice develop thrombocytopenia. (A) Expression of Sphk mRNA in MKs. Both Sphk1 and Sphk2 were detected in immature and mature MKs. M, DNA marker; +, with reverse transcriptase; –, without reverse transcriptase. Representative of 3 independent experiments. (B) Relative expression of Sphk1 or Sphk2 mRNA by fetal liver–derived mature (white) and immature MKs (black). Representative of 3 independent experiments performed in triplicate. (C) Platelet counts in peripheral blood from WT, Sphk1−/−, and Sphk2−/− mice on a Balb/c background (n = 6 for WT; n = 5 for Sphk1−/− mice; n = 7 for Sphk2−/− mice). (D) Platelet counts in peripheral blood from WT or Sphk2−/− on a C57Bl/6J background (n = 17 for WT; n = 9 for Sphk2−/− mice). (E) Platelet counts in peripheral blood from WT or Sphk2−/− BM chimeras on a C57Bl/6J background (n = 5 for WT chimeras; n = 7 for Sphk2−/− chimeras). All error bars represent standard error of the mean (SEM).
MKs express Sphk2 and Sphk2-deficient mice develop thrombocytopenia. (A) Expression of Sphk mRNA in MKs. Both Sphk1 and Sphk2 were detected in immature and mature MKs. M, DNA marker; +, with reverse transcriptase; –, without reverse transcriptase. Representative of 3 independent experiments. (B) Relative expression of Sphk1 or Sphk2 mRNA by fetal liver–derived mature (white) and immature MKs (black). Representative of 3 independent experiments performed in triplicate. (C) Platelet counts in peripheral blood from WT, Sphk1−/−, and Sphk2−/− mice on a Balb/c background (n = 6 for WT; n = 5 for Sphk1−/− mice; n = 7 for Sphk2−/− mice). (D) Platelet counts in peripheral blood from WT or Sphk2−/− on a C57Bl/6J background (n = 17 for WT; n = 9 for Sphk2−/− mice). (E) Platelet counts in peripheral blood from WT or Sphk2−/− BM chimeras on a C57Bl/6J background (n = 5 for WT chimeras; n = 7 for Sphk2−/− chimeras). All error bars represent standard error of the mean (SEM).
Normal MK development, serum thrombopoietin (Tpo) levels, platelet life span, and enhanced extramedullary thrombopoiesis in Sphk2−/− mice
To examine possible causes of thrombocytopenia in Sphk2−/− mice, we first performed CFU-MK assays to evaluate BM MK progenitors. We found that Sphk2−/− BM cells generated a similar number of CFU-MKs as WT cells (Figure 2A), suggesting that Sphk2−/− mice had a normal population of MK progenitors in the BM. Because BM MKs are the major source of platelet production, next we manually counted the number of MKs in femoral BM sections. However, histologic analysis revealed no significant difference in the number of BM MKs between Sphk2−/− and WT in vivo, excluding a reduced MK count as the cause of thrombocytopenia (Figure 2B-C). Besides the BM, the spleen acts as another source of platelet biogenesis, especially under pathophysiological conditions (eg, during thrombocytopenia). We therefore examined the spleens from 16- to 20-week-old Sphk2−/− mice by histologic analysis and detected more MKs in spleens from Sphk2−/− mice than from WT mice (Figure 2B,D). Moreover, the spleens of Sphk2−/− mice were much larger than WT (Figure 2E). This most likely results from extensive extramedullary hematopoiesis to partially compensate for the reduced number of circulating platelets. Endogenous hematopoietic stem and progenitor cells10 or circulating hematopoietic stem and progenitor cells11 may give rise to the MK lineage in the spleen. During MK maturation, MKs undergo polyploidization and increase their cytoplasmic size.12 In order to evaluate polyploidization, we measured DNA content in MKs by flow cytometry. Cytometric analysis showed that Sphk2−/− MKs displayed a similar percentage of polyploidic cells containing DNA contents >8N as WT (Figure 2F), suggesting that loss of Sphk2 does not affect the process of polyploidization in MKs. Because altered MK life span could influence thrombopoiesis,13 we measured MK life span in vitro. Our data showed that Sphk2−/− MKs displayed an identical life span to WT (supplemental Figure 1A). In addition, surface expression levels of CD41 and glykoprotein Ibalpha (GPIbα) in mature Sphk2−/− MKs were comparable to WT (supplemental Figure 1B-C). These results suggested that the maturation of Sphk2−/− MKs was not disturbed. We next analyzed serum Tpo levels, as Tpo is the principle physiological regulator of thrombopoiesis.14 However, the Tpo levels of Sphk2−/− mice were not altered (Figure 2G), thereby excluding a reduced Tpo level as a reason for thrombocytopenia in Sphk2−/− mice. Because the clearance of platelets also has an influence on homeostasis of circulating blood platelets,15 we next monitored platelet life span by in vivo biotin pulse labeling (for more details, see supplemental Materials and methods). We found that Sphk2−/− platelets had a similar life span as WT platelets (Figure 2H), which suggested that an increase in the clearance rate of platelets was also not responsible for thrombocytopenia in Sphk2−/− mice. Taken together, neither MK progenitors nor MK development nor the clearance of platelets was altered by loss of Sphk2.
Loss of Sphk2 results in extramedullary thrombopoiesis but does not change MK development, serum Tpo levels, or platelet life span. (A) Quantification of CFU-MK numbers in BM cells. Representative of 6 independent experiments performed in triplicate. (B) Representative immunostaining of MKs in mouse femoral BM and spleen. The samples were examined using a Leica microscope equipped with ×20 objective lens (numerical aperture [NA] = 0.5) or ×10 objective lens (NA = 0.3) and a commercial charge-coupled device (CCD) camera. Images were acquired by Axiovision software. MKs were detected by the MK-specific marker CD41 (green); 4,6-diamidino-2-phenylindole (blue); scale bar represents 10 µm. (C) Quantification of MK numbers per ×20 high-power field in femoral BM sections (n = 3 for each group). (D) Quantification of MK numbers per ×10 high-power field in spleen (n = 3 for each group). (E) The ratio of spleen weight to whole body weight (left) and the representative picture of extracted spleens from WT or Sphk2−/− mice (right) (n = 5 for each group; 16-20 weeks old). (F) The percentage of MKs with DNA ploidy (2N, 4N, and ≥8N) in WT and Sphk2−/− mice (n = 3 mice for each group). (G) Serum Tpo levels (n = 3 for each group). (H) Platelet life span of WT and Sphk2−/− mice (n = 3 for each group). All error bars represent SEM.
Loss of Sphk2 results in extramedullary thrombopoiesis but does not change MK development, serum Tpo levels, or platelet life span. (A) Quantification of CFU-MK numbers in BM cells. Representative of 6 independent experiments performed in triplicate. (B) Representative immunostaining of MKs in mouse femoral BM and spleen. The samples were examined using a Leica microscope equipped with ×20 objective lens (numerical aperture [NA] = 0.5) or ×10 objective lens (NA = 0.3) and a commercial charge-coupled device (CCD) camera. Images were acquired by Axiovision software. MKs were detected by the MK-specific marker CD41 (green); 4,6-diamidino-2-phenylindole (blue); scale bar represents 10 µm. (C) Quantification of MK numbers per ×20 high-power field in femoral BM sections (n = 3 for each group). (D) Quantification of MK numbers per ×10 high-power field in spleen (n = 3 for each group). (E) The ratio of spleen weight to whole body weight (left) and the representative picture of extracted spleens from WT or Sphk2−/− mice (right) (n = 5 for each group; 16-20 weeks old). (F) The percentage of MKs with DNA ploidy (2N, 4N, and ≥8N) in WT and Sphk2−/− mice (n = 3 mice for each group). (G) Serum Tpo levels (n = 3 for each group). (H) Platelet life span of WT and Sphk2−/− mice (n = 3 for each group). All error bars represent SEM.
Loss of Sphk2 has no effect on motility, localization, and size of MKs in vivo
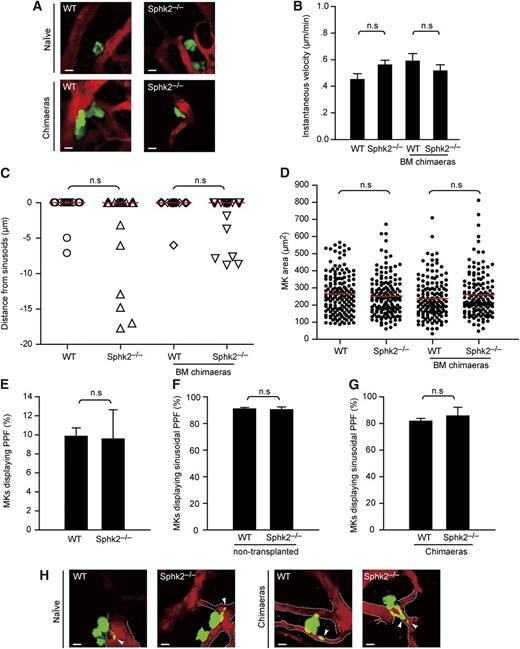
To investigate the behavior of MKs in vivo, we performed intravital multiphoton microcopy (MP-IVM) to directly visualize MKs in calvarian BM of WT × or Sphk2−/− × CD41-YFPki/+ mice, in which MKs and platelets express the yellow fluorescent protein (YFP) driven from the endogenous CD41 gene locus (Figure 3A; supplemental Movie 1).3 To visualize the bloodstream, we intravenously injected 2 MDa tetramethylrhodamine isothiocyanate-dextran, which enabled us to investigate BM sinusoidal vessels and their relationships to MKs (Figure 3A; supplemental Movie 1). As previously documented,3 we observed that WT MKs were large and sessile cells with extremely low motility (0.4-0.6 µm/min). There were no differences in motility between WT and Sphk2−/− naïve (nontransplanted) or BM chimeras, indicating that MK motility is not influenced by the loss of Sphk2 (Figure 3B).
Loss of Sphk2 has no effect on positioning, motility, or size of MKs or PP formation. (A) Representative in vivo images of YFP+ MKs (green) and vasculature (red) in mouse BM. Images were captured through a ×20 water immersion objective lens with NA = 0.95 using a BioTech TriM Scope system. WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ naïve (nontransplanted) mice (upper row); WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ BM chimeric mice (lower row). (B) Instantaneous lateral (x-y) velocity of MKs. Data were pooled from 3 mice per group. (C) Distance of MKs from BM sinusoids. Red lines, medians. Data were pooled from 3 mice in each group. (D) Surface area of MKs in femoral BM. Red lines, medians. Data were pooled from 3 mice per group. (E) The percentage of MKs displaying PP formation (PPF) (3 independent experiments performed in triplicate). (F) Intrasinusoidal PP formation in WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ naïve (nontransplanted) mice. MKs displaying intrasinusoidal PPF in vivo are presented as the percentage of all MKs carrying PPs (50-76 MKs per group, 3 independent experiments per genotype). (G) Intrasinusoidal PP formation in WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ BM chimeras mice. MKs displaying intrasinusoidal PPF in vivo represented as the percentage of all MKs displaying PPs (49 MKs per group, 3 independent experiments per genotypes). (H) Representative MP-IVM images of MKs with YFP+ PPs. Images were captured through a ×20 water immersion objective lens with NA = 0.95 using a BioTech TriM Scope system. Green, MKs and PP; red, sinusoids; arrowheads, intrasinusoidal YFP+ PPs. The dashed line highlights the sinusoids. All scale bars represent 20 µm. All error bars represent SEM.
Loss of Sphk2 has no effect on positioning, motility, or size of MKs or PP formation. (A) Representative in vivo images of YFP+ MKs (green) and vasculature (red) in mouse BM. Images were captured through a ×20 water immersion objective lens with NA = 0.95 using a BioTech TriM Scope system. WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ naïve (nontransplanted) mice (upper row); WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ BM chimeric mice (lower row). (B) Instantaneous lateral (x-y) velocity of MKs. Data were pooled from 3 mice per group. (C) Distance of MKs from BM sinusoids. Red lines, medians. Data were pooled from 3 mice in each group. (D) Surface area of MKs in femoral BM. Red lines, medians. Data were pooled from 3 mice per group. (E) The percentage of MKs displaying PP formation (PPF) (3 independent experiments performed in triplicate). (F) Intrasinusoidal PP formation in WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ naïve (nontransplanted) mice. MKs displaying intrasinusoidal PPF in vivo are presented as the percentage of all MKs carrying PPs (50-76 MKs per group, 3 independent experiments per genotype). (G) Intrasinusoidal PP formation in WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ BM chimeras mice. MKs displaying intrasinusoidal PPF in vivo represented as the percentage of all MKs displaying PPs (49 MKs per group, 3 independent experiments per genotypes). (H) Representative MP-IVM images of MKs with YFP+ PPs. Images were captured through a ×20 water immersion objective lens with NA = 0.95 using a BioTech TriM Scope system. Green, MKs and PP; red, sinusoids; arrowheads, intrasinusoidal YFP+ PPs. The dashed line highlights the sinusoids. All scale bars represent 20 µm. All error bars represent SEM.
MKs normally reside close to sinusoidal endothelium, a localization known to be of critical importance for proper platelet biogenesis in the BM.16 Hence, we next asked whether Sphk2 controlled the perivascular positioning of MKs in BM. Using MP-IVM, we observed that most MKs were located in close proximity to BM sinusoids in WT, as well as in Sphk2-deficient mice (Figure 3A; supplemental Movie 1). The median distances of all MKs from BM sinusoids showed no significant difference between WT and Sphk2−/− naïve mice, excluding abnormal positioning of MKs in vivo as a reason for thrombocytopenia (Figure 3C).
Finally, we evaluated the mean size of MKs in vivo using a histologic approach. Our results revealed that BM MKs from WT and Sphk2−/− mice were of similar size (Figures 2B and 3D). Collectively, our data suggest that neither migration nor the position of MKs nor their size in BM spaces is controlled by Sphk2.
Sphk2 is dispensable for PP formation
The previous findings demonstrate that Sphk2 deletion has no effects either on general MK development or on the clearance of platelets. This prompted us to investigate whether Sphk2 is required for the terminal stage of thrombopoiesis, in which MKs release platelets into the bloodstream. The mechanism of platelet release from MKs has been shown to follow the prevalent model of thrombopoiesis, the PP model. We therefore cultured fetal liver cell–derived MKs and evaluated the efficiency of PP formation by WT and Sphk2−/− MKs in vitro. Our results show that there is no significant difference in the efficiency of PP formation between Sphk2−/− and WT MKs in vitro (Figure 3E). Of note is that the efficiency of PP formation in WT MKs in our studies is lower than some previous studies,17 which most likely results from different culture conditions (see supplemental Materials and methods). Moreover, Sphk2−/− MKs extended long PPs with intermediate swellings and platelet-like tips, similar to WT MKs (supplemental Figure 1D). The morphology of Sphk2−/− PPs was comparable to WT at the final stage, regarding the length of PPs, the thickness of PP stems, and the diameter of PP tips (supplemental Figure 1E-G). Our data showed that Sphk2−/− MKs had no defect in PP formation.
To further examine whether PP formation or PP-like cytoplasmic protrusions existed in vivo, we used MP-IVM to visualize MKs in WT × CD41-YFPki/+ and Sphk2−/− × CD41-YFPki/+ mice. We detected long cytoplasmic protrusions, which were similar to the structures of PPs in vitro, in both WT × CD41-YFPki/+ and Sphk2−/− × CD41-YFPki/+ mice in vivo (Figure 3F-H; supplemental Movie 2), suggesting that Sphk2−/− MKs had no defects in PP formation or PP-like protrusions in vivo. Our previous studies have shown that most PPs were polarized into BM sinusoid vessels and that aberrant PP formation in the BM interstitium resulted in a defective platelet production.7 To investigate the role of Sphk2 for polarization of PPs in BM, we applied MP-IVM in WT or Sphk2−/− × CD41-YFPki/+ mice (Figure 3F-H; supplemental Movie 2). Our results revealed that most Sphk2−/− MKs in both naïve (nontransplanted) and BM chimeric mice predominantly extended PPs into BM sinusoidal vessels, similar to WT MKs (Figure 3F-H; supplemental Movie 2). This excluded a potential influence of Sphk2 deficiency on polarized PP formation and ruled out the possibility that aberrant PP formation inside the interstitium contributes to thrombocytopenia in Sphk2−/− mice. Together, applying in vitro assays and in vivo MP-IVM, our findings demonstrate that Sphk2 is dispensable for PP formation and extension into the blood.
Sphk2−/− MKs display a defect in PP fragmentation
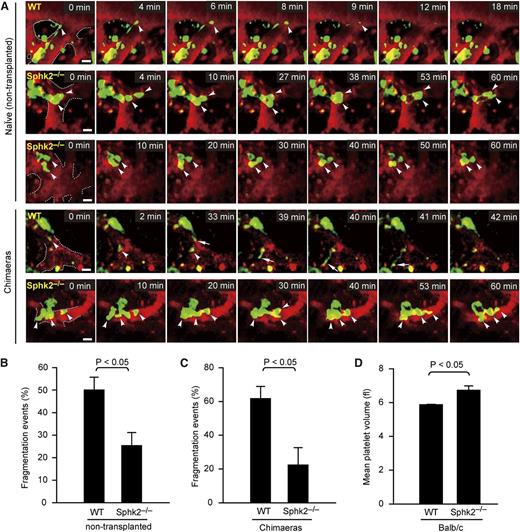
The previous data excluded defects in PP formation during terminal differentiation of Sphk2−/− MKs. Therefore, the terminal steps of PP maturation (ie, PP fragmentation and shedding) were examined in more detail. To define the role of Sphk2 for fragmentation of PPs in vivo, we monitored PP fragmentation events from MKs in real time using MP-IVM. We observed that WT MKs continually extended cytoplasmic extensions into the bloodstream and promptly released the tips of PPs into BM sinusoids. The frequency of PP fragmentation or shedding in MKs displaying PPs was 50% ± 10.00% (mean ± standard deviation) in Sphk2+/+ × CD41-YFPki/+ mice (Figure 4A-C; supplemental Movie 3). In contrast, we observed that Sphk2−/− × CD41-YFPki/+ MKs often retracted PPs, instead of continuously extending and releasing PPs into BM sinusoids. General loss of Sphk2 significantly reduced PP fragmentation or shedding by 50% in vivo (Figure 4A-B; supplemental Movie 3). Loss of Sphk2 exclusively in the hematopoietic system also resulted in a defect in PP shedding (Figure 4A,C; supplemental Movie 3), ruling out an influence of Sphk2 deficiency in nonhematopoietic systems. We observed that the defect in PP shedding led to irregular and clumsy cytoplasmic PP extensions in Sphk2-deficient mice (Figure 4A; supplemental Movie 3). These results clearly demonstrate that Sphk2 is required for proper PP fragmentation in vivo.
Sphk2 deficiency results in inefficient PP fragmentation in vivo. (A) Role for Sphk2 in PP shedding in vivo visualized by MP-IVM. WT MKs frequently shed PPs as shown in the first and fourth rows from top to bottom. Loss of Sphk2 abolishes PP shedding as shown in the second, third, and fifth rows from top to bottom. Images were captured through a ×20 water immersion objective lens with NA = 0.95 using a LaVision BioTech TriM Scope system. Arrowheads and arrows indicate the tips of PPs. Green, MKs and PP; red, sinusoids. All scale bars represent 20 µm; time in minutes. The dashed line highlights the sinusoids. (B) Percentage of PP fragmentation events observed by MP-IVM over 1 hour in WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ naïve (nontransplanted) mice. Data are pooled from 3 to 5 independent experiments from each group (n = 21-43 per group). (C) Percentage of PP fragmentation events observed by MP-IVM over 1 hour in WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ chimeras. Data are pooled from 3 to 4 independent experiments from each group (n = 21-31 per group). (D) MPV in peripheral blood from WT and Sphk2−/− mice on a Balb/c background (n = 9 for WT; n = 6 for Sphk2−/− mice). All error bars represent SEM.
Sphk2 deficiency results in inefficient PP fragmentation in vivo. (A) Role for Sphk2 in PP shedding in vivo visualized by MP-IVM. WT MKs frequently shed PPs as shown in the first and fourth rows from top to bottom. Loss of Sphk2 abolishes PP shedding as shown in the second, third, and fifth rows from top to bottom. Images were captured through a ×20 water immersion objective lens with NA = 0.95 using a LaVision BioTech TriM Scope system. Arrowheads and arrows indicate the tips of PPs. Green, MKs and PP; red, sinusoids. All scale bars represent 20 µm; time in minutes. The dashed line highlights the sinusoids. (B) Percentage of PP fragmentation events observed by MP-IVM over 1 hour in WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ naïve (nontransplanted) mice. Data are pooled from 3 to 5 independent experiments from each group (n = 21-43 per group). (C) Percentage of PP fragmentation events observed by MP-IVM over 1 hour in WT × CD41-YFPki/+ or Sphk2−/− × CD41-YFPki/+ chimeras. Data are pooled from 3 to 4 independent experiments from each group (n = 21-31 per group). (D) MPV in peripheral blood from WT and Sphk2−/− mice on a Balb/c background (n = 9 for WT; n = 6 for Sphk2−/− mice). All error bars represent SEM.
Given that PP fragmentation into distinct platelets continues in the circulation, we predicted that the failure to efficiently shed PPs in BM vasculature also results in a defect in PP fragmentation in the systemic circulation and that inefficiently fragmented PPs could possibly be observed occluding the pulmonary microcirculation. To test this, we examined the lungs from WT and Sphk2−/− mice ex vivo using MP-IVM. Indeed, we detected more and longer YFP+ fragments occluding the lungs from Sphk2−/− × CD41-YFPki/+ mice than from WT controls (supplemental Figure 2A,C-D; supplemental Movie 4). These YFP+ fragments excluded a nuclear stain (supplemental Figure 2B), implying that they were indeed fragments from MKs, instead of the primary MK body. A similar phenotype was also observed in Sphk2−/− BM chimeras (supplemental Figure 2A,C,E; supplemental Movie 4), excluding the possibility that an aberrant pulmonary vasculature was responsible for the presence of PPs in lungs in the absence of Sphk2. As we reported previously,7 a defect in PP fragmentation can result in larger platelets in peripheral blood. Indeed, mean platelet volume (MPV) was significantly higher in Sphk2−/− mice on a Balb/c background, as compared with WT mice (Figure 4D). However, we did not observe significantly higher MPV in Sphk2 mutants on a C57Bl/6J background (supplemental Tables 2 and 3). It has been well established that the genetic background of mouse line significantly affects platelet size (see the Jackson Laboratory Mouse Phenome Database).18 The differences in platelet size between WT and Sphk2−/− mice on a Balb/c background might be more pronounced than those on a C57Bl/6J background; thus, MPV measurement is sensitive enough to resolve the differences in the former mice but not in the latter. Nevertheless, our intravital observation enabled us to monitor the whole process of PP fragmentation at the single MK level and revealed an obvious defect in PP fragmentation in Sphk2−/− MKs.
Extracellular S1P does not rescue the defect of Sphk2−/− MKs in PP fragmentation
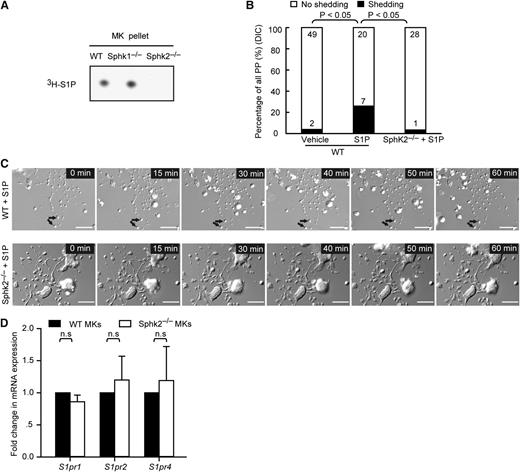
We next addressed how Sphk2 modulates PP fragmentation. On the mRNA level, Sphk2 is the major Sphk isoform expressed by mature MKs (Figure 1B). We therefore next tested the contribution of Sphk2 to overall S1P synthesis in MKs and examined S1P generation by WT, Sphk1−/−, and Sphk2−/− MKs. Loss of Sphk1 did not change S1P synthesis by MKs, whereas loss of Sphk2 significantly reduced megakaryocytic S1P production (Figure 5A). This indicates that Sphk2 contributes to the major fraction of S1P in MKs. We have previously shown that extracellular S1P controls thrombopoiesis via its megakaryocytic membrane receptor S1pr1.7 Hence, we hypothesized that Sphk2 might control PP fragmentation in an autocrine and/or paracrine manner by synthesizing and releasing S1P into the MK microenvironment. To address this, we incubated MKs at the same developmental stage, when MKs developed long PPs with intermediate swellings and platelet-like tips, with extracellular S1P and monitored PP fragmentation by time-lapse video differential interference contrast (DIC) microscopy. Our findings show that extracellular S1P triggered PP fragmentation and shedding in WT MKs in vitro (Figure 5B-C; supplemental Movie 5), as we previously observed.7 Surprisingly, however, extracellular S1P did not rescue the defect in PP fragmentation of Sphk2−/− MKs in vitro (Figure 5B-C; supplemental Movie 5). Of note, S1P significantly decreased the size of PP tips in WT MKs (supplemental Figure 1G), which might result from S1P-induced retraction, as observed in other cell types.19-21 Surprisingly, S1P treatment resulted in enlarged PP tips on Sphk2−/− MKs (supplemental Figure 1G), reminiscent of the larger platelets in Sphk2−/− mice on a Balb/c background (Figure 4D), given that the size of PP tips may account for the terminal platelet size.22 This response to extracellular S1P was not due to differences in the expression profile of S1P receptors in Sphk2−/− compared with WT MKs (Figure 5D). Taken together, these findings rule out the possibility that Sphk2 regulates PP fragmentation in a paracrine manner through extracellular S1P release by MKs; rather, it functions intracellularly in an autocrine fashion. This conclusion is also supported by the fact that the increased S1P plasma levels observed in Sphk2−/− mice do not correct the defect in PP fragmentation in vivo (Figure 4A-C).23
Extracellular S1P cannot rescue the defect of Sphk2−/− MKs in PP fragmentation. (A) Representative autoradiogram of 3H-S1P in WT, Sphk1−/−, and Sphk2−/− MKs. (B) The number of PPs with or without fragmentation observed by DIC microscopy in vitro over 1 hour in the indicated groups. Data are pooled from 7 to 10 independent experiments from each group (n = 27-51 per group). (D) DIC microscopic analysis of PP shedding in the presence of 10 µM S1P. MKs were kept on a heated microincubator to keep the temperature at 37°C in serum-free medium and monitored using a DIC microscope system equipped with a ×40 oil objective lens with NA = 0.7. Arrow, platelets released from PP stems. WT MKs (upper row); Sphk2−/− MKs (lower row). (D) Fold change of S1pr1, S1pr2, and S1pr4 mRNA by fetal liver–derived mature Sphk2−/− MKs (white) compared with mature WT MKs (black). The mRNA levels were normalized to Gapdh. Representative of 3 independent experiments performed in triplicate. All scale bars represent 20 µm; time in minutes. All error bars represent SEM.
Extracellular S1P cannot rescue the defect of Sphk2−/− MKs in PP fragmentation. (A) Representative autoradiogram of 3H-S1P in WT, Sphk1−/−, and Sphk2−/− MKs. (B) The number of PPs with or without fragmentation observed by DIC microscopy in vitro over 1 hour in the indicated groups. Data are pooled from 7 to 10 independent experiments from each group (n = 27-51 per group). (D) DIC microscopic analysis of PP shedding in the presence of 10 µM S1P. MKs were kept on a heated microincubator to keep the temperature at 37°C in serum-free medium and monitored using a DIC microscope system equipped with a ×40 oil objective lens with NA = 0.7. Arrow, platelets released from PP stems. WT MKs (upper row); Sphk2−/− MKs (lower row). (D) Fold change of S1pr1, S1pr2, and S1pr4 mRNA by fetal liver–derived mature Sphk2−/− MKs (white) compared with mature WT MKs (black). The mRNA levels were normalized to Gapdh. Representative of 3 independent experiments performed in triplicate. All scale bars represent 20 µm; time in minutes. All error bars represent SEM.
Sphk2 regulates SFK expression and activity in MKs
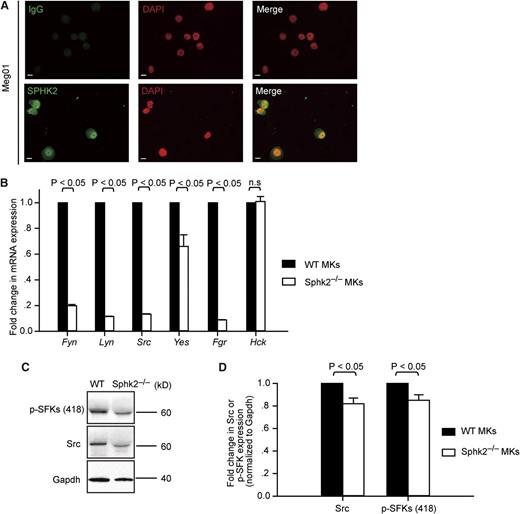
Recent investigations revealed that endogenous Sphk2 and nuclear S1P regulate histone deacetylation complexes (HDAC) 1 and 2 and therefore modulate gene expression.24 Because of the lack of appropriate antibodies against murine SPHK2, to examine the subcellular localization of Sphk2 in MKs, we performed immunostaining of SPH2K in a human MK cell line, Meg01, using an anti-human SPHK2 antibody. Interestingly, our immunostaining showed that SPHK2 was primarily localized to the nucleus of MKs, as reported previously in other cell types (Figure 6A).25 This indicated that Sphk2 could be involved in the regulation of gene expression during MK maturation. Given that SFKs are critical for thrombopoiesis,26,27 we examined whether Sphk2 influences SFK gene expression in MKs. The family of SFKs consists of 8 members, including Lyn, Hck, Lck, Blk, Src, Fyn, Yes, and Fgr.28 In agreement with previous reports,29 we detected mRNA encoding for 6 SFK members (Fyn, Lyn, Src, Yes, Fgr, and Hck) in MKs (Figure 6B). Although Hck mRNA expression in Sphk2−/− MKs was comparable to WT MKs, mRNA expression of the other SFK members, Fyn, Lyn, Src, Yes, and Fgr, was significantly reduced in Sphk2−/− MKs by 81%, 89%, 87%, 34%, and 92%, respectively (Figure 6B). Considering that MKs express Hck only at a low level and that other SFKs are expressed at higher levels,29 we predicted that the overall SFK activity was significantly limited in Sphk2−/− MKs. To further evaluate this, we performed western blot analysis to determine total SFK activity in MKs. Consistent with the reduced SFK gene expression, our data show that total Src protein levels were significantly reduced in Sphk2−/− MKs (Figure 6C-D). We further evaluated SFK activity by detecting the activation loop phospho-tyrosine-418, termed p-SFKs (418), in SFKs. We observed that the overall SFK activity was also significantly reduced in Sphk2−/− MKs.
Sphk2 regulates SFK expression and activity in MKs. (A) Expression of SPHK2 in human megakaryocytic cell lines, Meg01. The samples were examined using a Leica microscope equipped with ×20 objective lens (NA = 0.5) and a commercial CCD camera. Images were acquired by Axiovision software. Green, SPHK2 or IgG; red, 4′,6-diamidino-2-phenylindole. Scale bar represents 10 µm. All cells examined stained positive for SPHK2. (B) Fold change of Fyn, Lyn, Src, Yes, Fgr, and Hck mRNA by BM-derived mature Sphk2−/− MKs (white) compared with mature WT MKs (black). The mRNA levels were normalized to Gapdh. Representative of 3 independent experiments performed in triplicate. (C) Western blot analysis of Src and p-SFKs in WT or Sphk2−/− MKs. Gapdh served as loading control. Representative of 5 independent experiments. (D) Fold change of Src and p-SFKs (418) levels in BM-derived mature Sphk2−/− MKs (white) compared with mature WT MKs (black). The Src and p-SFKs levels were quantified by western blot. The density of Src or p-SFKs was normalized to Gapdh. Data were pooled from 3 to 4 independent experiments. All error bars represent SEM.
Sphk2 regulates SFK expression and activity in MKs. (A) Expression of SPHK2 in human megakaryocytic cell lines, Meg01. The samples were examined using a Leica microscope equipped with ×20 objective lens (NA = 0.5) and a commercial CCD camera. Images were acquired by Axiovision software. Green, SPHK2 or IgG; red, 4′,6-diamidino-2-phenylindole. Scale bar represents 10 µm. All cells examined stained positive for SPHK2. (B) Fold change of Fyn, Lyn, Src, Yes, Fgr, and Hck mRNA by BM-derived mature Sphk2−/− MKs (white) compared with mature WT MKs (black). The mRNA levels were normalized to Gapdh. Representative of 3 independent experiments performed in triplicate. (C) Western blot analysis of Src and p-SFKs in WT or Sphk2−/− MKs. Gapdh served as loading control. Representative of 5 independent experiments. (D) Fold change of Src and p-SFKs (418) levels in BM-derived mature Sphk2−/− MKs (white) compared with mature WT MKs (black). The Src and p-SFKs levels were quantified by western blot. The density of Src or p-SFKs was normalized to Gapdh. Data were pooled from 3 to 4 independent experiments. All error bars represent SEM.
SFK activity is required for PP fragmentation
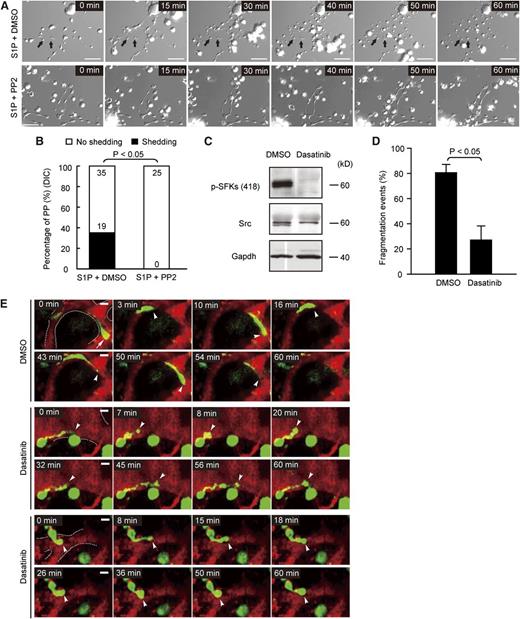
To test if SFK activity is required for S1P-induced PP fragmentation, we used 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) to inhibit SFK activity in vitro and monitored S1P-triggered PP fragmentation by time-lapse video DIC microscopy. We observed that the inhibition of SFK activity completely blocked S1P-induced PP fragmentation in WT MKs in vitro (Figure 7A-B; supplemental Movie 6). In a recent study, it has been shown that the cancer drug dasatinib inhibits SFK activity in MKs, resulting in thrombocytopenia in vivo.27 We therefore determined here whether inhibition of SFK activity by dasatinib leads to a defect in PP fragmentation and shedding similar to the one observed in Sphk2−/− MKs (Figure 4A-C; supplemental Movie 3). Consistent with the previous study,27 dasatinib completely blocked SFK activity in MKs (Figure 7C). Intravital MP-IVM revealed that dasatinib significantly retarded PP fragmentation in vivo (Figure 7D-E; supplemental Movie 7). The failure to shed PPs resulted in similar irregular and clumsy cytoplasmic PP extensions (Figure 7E; supplemental Movie 7) as we observed in Sphk2-deficient mice (Figure 4A; supplemental Movie 3). These results indicate that SFK activity is required for efficient PP fragmentation and the shedding of platelets into the bloodstream.
SFK activity is required for PP fragmentation in vivo. (A) DIC microscopy image sequences of PPs in vitro. Arrow, platelets released from PP stems. MKs were kept on a heated microincubator to keep the temperature at 37°C in serum-free medium and monitored using a DIC microscope system equipped with a ×40 oil objective lens with NA = 0.7. MKs treated with 10 µM S1P and dimethylsulfoxide (DMSO) (as vehicle for PP2) (upper row); MKs treated with 10 µM S1P and 10 µM PP2 (lower row). (B) S1P-induced PP fragmentation is dependent on SFK activity. The number of PPs with or without fragmentation observed by DIC microscopy in vitro over 1 hour in the indicated groups. Data are pooled from 5 to 7 independent experiments from each group (n = 25-54 per group). (C) Western blot analysis of Src and p-SFKs in WT MKs treated with vehicle (DMSO) or 10 µM dasatinib. Gapdh served as loading control. Representative of 3 independent experiments. (D) Percentage of PP fragmentation events observed by MP-IVM over 1 hour in the indicated groups. Data are pooled from 4 to 5 independent experiments from each group (n = 32-33 per group). (E) Role of SFKs in PP shedding in vivo visualized by MP-IVM. Dasatinib abolishes PP shedding in vivo (middle 2 and lower 2 rows). Images were captured through a ×20 water immersion objective lens with NA = 0.95 using a BioTech TriM Scope system. Arrowheads and arrows indicate the tips of PPs. Green, MKs and PP; red, sinusoids. The dashed line highlights the sinusoids. All scale bars represent 20 µm; time in minutes. All error bars represent SEM.
SFK activity is required for PP fragmentation in vivo. (A) DIC microscopy image sequences of PPs in vitro. Arrow, platelets released from PP stems. MKs were kept on a heated microincubator to keep the temperature at 37°C in serum-free medium and monitored using a DIC microscope system equipped with a ×40 oil objective lens with NA = 0.7. MKs treated with 10 µM S1P and dimethylsulfoxide (DMSO) (as vehicle for PP2) (upper row); MKs treated with 10 µM S1P and 10 µM PP2 (lower row). (B) S1P-induced PP fragmentation is dependent on SFK activity. The number of PPs with or without fragmentation observed by DIC microscopy in vitro over 1 hour in the indicated groups. Data are pooled from 5 to 7 independent experiments from each group (n = 25-54 per group). (C) Western blot analysis of Src and p-SFKs in WT MKs treated with vehicle (DMSO) or 10 µM dasatinib. Gapdh served as loading control. Representative of 3 independent experiments. (D) Percentage of PP fragmentation events observed by MP-IVM over 1 hour in the indicated groups. Data are pooled from 4 to 5 independent experiments from each group (n = 32-33 per group). (E) Role of SFKs in PP shedding in vivo visualized by MP-IVM. Dasatinib abolishes PP shedding in vivo (middle 2 and lower 2 rows). Images were captured through a ×20 water immersion objective lens with NA = 0.95 using a BioTech TriM Scope system. Arrowheads and arrows indicate the tips of PPs. Green, MKs and PP; red, sinusoids. The dashed line highlights the sinusoids. All scale bars represent 20 µm; time in minutes. All error bars represent SEM.
Discussion
Our present data suggest that the bioactive lipid S1P acts as an intracellular mediator in MKs that intrinsically controls platelet biogenesis at least in part by regulating expression and activity of SFKs. We found that the loss of the major Sphk isoenzyme in MKs, Sphk2, but not Sphk1 resulted in impaired intracellular S1P synthesis in the megakaryocytic lineage. Both a general and a hematopoietic cell-specific deficiency of Sphk2 resulted in thrombocytopenia, whereas mice lacking Sphk1 had normal blood platelet counts. A high concentration of extracellular S1P did not rescue the defect of Sphk2−/− MK in PP fragmentation, suggesting that Sphk2 predominantly controls platelet biogenesis by producing intracellular S1P.
Our study identifies SFKs as a major downstream target of Sphk2 and intracellular S1P. Indeed, genetic ablation of Sphk2 resulted in reduced expression and activity of SFKs, a group of kinases that are essential for platelet biogenesis.26,27 We found that Sphk2 predominantly localized in the MK nucleus. It has been reported that Sphk2 plays a role in epigenetic regulation of gene expression.24 Hait et al24 demonstrated that Sphk2 is associated in the nucleus with HDAC1 and HDAC2 in repressor complexes and that Sphk2 produces nuclear S1P to inhibit HDAC1 and HDAC2 activity, leading to the epigenetic regulation of genes such as cyclin-dependent kinase inhibitor p21 and the transcriptional regulator c-fos. A similar regulation likely explains our observation that expression of SFKs is reduced in Sphk2 null mice. Notably, pharmacologic blockade of SFK activity by dasatinib mimics the effects of a genetic loss of Sphk2. Hence, we conclude that the molecular mechanism explaining how Sphk2 controls thrombopoiesis is most likely related to the expression and activity of SFKs modulated via intracellular S1P.
We have previously reported that, in addition to the role for intracellular S1P identified here, S1P in the extracellular milieu also plays an essential role in the control of thrombopoiesis acting via its receptor S1pr1.7 Consistent with other reports,30-34 we have reported that the interstitial S1P concentrations in the BM are much lower than the S1P concentrations in the blood,7 resulting in a steep transendothelial S1P gradient in BM. This transendothelial S1P gradient in the BM controls the directionality of PP formation and elongation via its receptor S1pr1. Subsequently, intrasinusoidal PPs encounter high concentrations of S1P, which enhances intravascular PP shedding. Correspondingly, mice lacking S1pr1 expression on MKs develop severe thrombocytopenia.7 Hence, our present manuscript together with our previous data indicate that extra- and intracellular S1P play additive roles in physiological thrombopoiesis and critically control the final steps of platelet biogenesis, that is, directional PP formation (extracellular S1P) and PP shedding (extracellular and intracellular S1P) (supplemental Figure 2F). Our previous investigations demonstrated that S1P-mediated Rac activation was involved in polarization of PPs.7 It is well established that the Rac guanosine triphosphatase (GTPase) plays an essential role in polarized cell protrusion and directional migration.35 The transendothelial S1P gradient most likely leads to a polarized distribution of Rac with high activity at the PP tips and thus induces PP protrusion into the bloodstream. The intravascular PPs are further dissociated from MKs and fragmented into the blood. It is widely accepted that PP fragmentation and shedding is passively controlled by shear stress in the blood.3 However, our results support a new model, in which the shedding of PPs is controlled not only by hydrodynamic forces, but also through intracellular signaling pathways. Our investigations showed that both Rac and SFKs serve as key biological regulators in this process.7 Notably, Rac GTPase and SFK signaling pathways mutually regulate each other.36 On one hand, it has been reported that Rac GTPase mediates the translocation of Src from perinuclear regions to the plasma membrane.37 On the other hand, SFK activation has been shown to modulate the activity of Rho-family GTPases by regulating guanine nucleotide-exchanger factors, including Tiam-1, Vav, and PIX, to increase Rac activity.38 This interplay between SFKs and Rac GTPase might be crucial for platelet biogenesis by rearranging the cytoskeleton in a spatiotemporal manner (supplemental Figure 2F).
Our data also have clinical implications. Dasatinib is a potent inhibitor of BCR-ABL1 and SFKs with established efficacy in patients with imatinib-resistant chronic myeloid leukemia in chronic phase and is more effective than high-dose imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase.39 However, thrombocytopenia was more frequently observed in patients treated with dasatinib than in patients receiving imatinib.40 The mechanism of dasatinib-induced thrombocytopenia has been unclear until recently. However, we have previously reported that dasatinib results in impaired migration of MKs.27 Here, we show that inhibition of SFKs by dasatinib retards PP fragmentation and shedding. Hence, we now provide an additional explanation for dasatinib-induced thrombocytopenia in patients, extending our previous findings.27
Collectively, our results support a model whereby Sphk2 is required for efficient PP fragmentation, related to reduced SFK expression and activity, and suggest that Sphk2-synthesized intracellular S1P is required for proper thrombopoiesis. Inefficient PP fragmentation and shedding leads to the reduced platelet production and thrombocytopenia in mice lacking Sphk2. These data have important implications for the study of thrombopoiesis and provide a new understanding of the physiology of thrombopoiesis. Strategies to modulate intracellular S1P, Sphk2, and SFK activity may provide new approaches to increase the efficiency of platelet production and could lead to the development of a new therapy to treat thrombocytopenia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Andreas Billich for supplying Sphk1−/−, Sphk2−/−, and corresponding WT control mice.
This study was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 914) (S.M.) and FP7 project PRESTIGE (S.M.).
Authorship
Contribution: L.Z., K.R.L., M.L., and S.M. conceived of and designed the study and wrote the manuscript; L.Z. performed MP-IVM, generated chimeras, and performed in vitro MK assays; N.U. performed S1P synthesis assay; F.G. helped with in vitro shedding assay; T.P. performed in vivo experiments; and A.M. and S.P.W. helped with data interpretation and discussion.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steffen Massberg, Medizinische Klinik und Poliklinik I, Klinikum der Universität, LMU, Marchioninistrasse 15, 81377 Munich, Germany; e-mail: steffen.massberg@med.uni-muenchen.de.

![Figure 2. Loss of Sphk2 results in extramedullary thrombopoiesis but does not change MK development, serum Tpo levels, or platelet life span. (A) Quantification of CFU-MK numbers in BM cells. Representative of 6 independent experiments performed in triplicate. (B) Representative immunostaining of MKs in mouse femoral BM and spleen. The samples were examined using a Leica microscope equipped with ×20 objective lens (numerical aperture [NA] = 0.5) or ×10 objective lens (NA = 0.3) and a commercial charge-coupled device (CCD) camera. Images were acquired by Axiovision software. MKs were detected by the MK-specific marker CD41 (green); 4,6-diamidino-2-phenylindole (blue); scale bar represents 10 µm. (C) Quantification of MK numbers per ×20 high-power field in femoral BM sections (n = 3 for each group). (D) Quantification of MK numbers per ×10 high-power field in spleen (n = 3 for each group). (E) The ratio of spleen weight to whole body weight (left) and the representative picture of extracted spleens from WT or Sphk2−/− mice (right) (n = 5 for each group; 16-20 weeks old). (F) The percentage of MKs with DNA ploidy (2N, 4N, and ≥8N) in WT and Sphk2−/− mice (n = 3 mice for each group). (G) Serum Tpo levels (n = 3 for each group). (H) Platelet life span of WT and Sphk2−/− mice (n = 3 for each group). All error bars represent SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/122/5/10.1182_blood-2012-12-473884/4/m_791f2.jpeg?Expires=1763474720&Signature=kxumFDB-EnvIqZcNabifqJZfZmf-r~ucWiZHVR1ViCg2XDtJKBni66RbyzSm4okoOHEQX-B77zga95GNFDW7WlMweq-i3yWQs3UAy3ImEqoENf4mnLK2fJ8YX5wQNO5Tqp7NDF-TV20Lr0e5OYWkGI9oylamhiLma8x48R70hLyt8sCozpZ3x8daMr1Z8lnwaiIgCPlSfdUeM53hc6y1pDtzJtwh07AMN1W49WO43BN8uvD4edaejtabJrEU8fCetC64tVSmxe~6Z75KZfNppgSt8Og~hN2XQr8tFYP6C6ksCQ7BYV-TC8zFKRiWO7MHEnXyKHYRaN2V5n0Un9nFMw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)