Key Points
AAV-mediated gene transfer of vascular growth factors.
Among 7 endothelial growth factors, PlGF has optimal properties for induction of new vessel growth without vascular leakage or inflammation in the brain.
Abstract
Vascular bypass procedures in the central nervous system (CNS) remain technically challenging, hindered by complications and often failing to prevent adverse outcome such as stroke. Thus, there is an unmet clinical need for a safe and effective CNS revascularization. Vascular endothelial growth factors (VEGFs) are promising candidates for revascularization; however, their effects appear to be tissue-specific and their potential in the CNS has not been fully explored. To test growth factors for angiogenesis in the CNS, we characterized the effects of endothelium-specific growth factors on the brain vasculature and parenchyma. Recombinant adeno-associated virus (AAV) vectors encoding the growth factors were injected transcranially to the frontoparietal cerebrum of mice. Angiogenesis, mural cell investment, leukocyte recruitment, vascular permeability, reactive gliosis and neuronal patterning were evaluated by 3-dimensional immunofluorescence, electron microscopy, optical projection tomography, and magnetic resonance imaging. Placenta growth factor (PlGF) stimulated robust angiogenesis and arteriogenesis without significant side effects, whereas VEGF and VEGF-C incited growth of aberrant vessels, severe edema, and inflammation. VEGF-B, angiopoietin-1, angiopoietin-2, and a VEGF/angiopoietin-1 chimera had minimal effects on the brain vessels or parenchyma. Of the growth factors tested, PlGF emerged as the most efficient and safe angiogenic factor, hence making it a candidate for therapeutic CNS revascularization.
Introduction
Angiogenesis is the vascular growth factor–promoted formation of new blood vessels from preexisting vasculature. Therapeutic angiogenesis via gene vector delivery has been successfully used to increase blood perfusion in skeletal and heart muscle in a variety of animal models,1,2 although proangiogenic therapies have not yet been approved for clinical use. Vascular endothelial growth factors (VEGFs), comprising VEGF (or VEGF-A), VEGF-B, placenta growth factor (PlGF), VEGF-C and VEGF-D, and the angiopoietins (Ang1, Ang2, Ang3/4), act primarily on endothelial cells, making VEGFs attractive candidates for proangiogenic therapies. The proangiogenic effects of vascular growth factors are apparently species and tissue-specific3-8 ; thus the optimal choice of growth factor may vary depending on the target tissue.
Cerebral hypoperfusion acutely may elicit stroke, and chronically leads to cognitive impairment,9 possibly preceding vascular dementia.10 The elderly are more prone to cerebral hypoperfusion,10 although chronic cerebral hypoperfusion may affect the young in rare conditions.11 Therapeutic options are limited and one of the most common revascularization procedures in the central nervous system (CNS) is direct extracranial-intracranial (EC-IC) vessel bypass surgery, which is technically challenging and often clinically inefficient.12,13 Furthermore, it creates an abrupt increase in the cerebral blood flow, risking the rare, but potentially life-threatening, hyperperfusion syndrome.14 The development of a safe and technically robust CNS revascularization strategy could benefit patients suffering from common neurovascular diseases, such as vascular dementia and stroke.
Despite the obvious clinical demand for novel strategies of CNS revascularization, systematic studies of long-term angiogenic effects of vascular growth factors in the CNS have not been carried out. A tissue-specific requirement for proangiogenic therapy in the CNS is that the treatment should not interfere with the highly selective blood-brain barrier (BBB). We screened the properties of vessels formed upon expression of VEGFs, angiopoietins, and a chimeric VEGF/Ang1 growth factor via an efficient and minimally immunogenic viral gene transfer vector injected directly into the murine CNS. Our results implicate PlGF as a potent angiogenic factor in the CNS with minimal adverse effects for future clinical trials involving patients suffering from chronic cerebral hypoperfusion.
Methods
All mouse experiments were approved by the Provincial State Office of Southern Finland and carried out in accordance with the institutional guidelines.
Generation of adeno-associated virus vectors
The recombinant adeno-associated virus (AAV) vectors of serotype 9 were produced as described previously.1 Briefly, human or mouse vascular growth factors were placed under the cytomegalovirus promoter in pSubCMV-WPRE plasmid vector, suitable for AAV production.15 The AAVs were produced by three-plasmid transfection method, and purified as described by Zolotukhin et al.16 To generate AAV of serotype 2, we used the pDG helper plasmid,17 and to generate AAV8 and AAV9, we used a combination of pBS-E2A-VA-E4 and either p5e18-VD2/8 or p5e18-VD2/9 helper plasmids, respectively.18,19 All viral preps were quantified by real-time–polymerase chain reaction and tested in vitro for their transduction efficiencies.
Generation of the chimeric vascular growth factor, VA1
The chimeric vascular growth factor, VA1, has been described previously.20 VA1 consists of the receptor-binding regions of VEGF and Ang1 fused in the same orientation, hence binding VEGF receptor-1 (VEGFR-1) or VEGF receptor-2 (VEGFR-2) and Tie2 simultaneously. The therapeutic potential of VA1 was validated in a rodent model of hind-limb ischemia.20
Intracranial subcortical injection of the AAV vectors
Six- to 7-week-old female C57BL/6J mice (3 per group) were anesthetized by intraperitoneal injections of xylazine (10 mg kg−1; Bayer) and ketamine (80 mg kg−1; Pfizer). Via an ∼1-cm–long sagittal scalp incision, rostral and caudal from the coronal suture in the paramedian line (supplemental Figure 1A, available on the Blood website), 4 separate injections of 3 × 109 AAV9 particles encoding human VEGF, different isoforms of human or mouse VEGF-B (hVEGF-B or mVEGF-B), human or mouse PlGF-2 (hPlGF or mPlGF),21 human VEGF-C,1,22 chimeric cartilage oligomeric matrix protein-angiopoietin 1 (cAng1),23 mouse Ang2, VA1,20 or human serum albumin (HSA) (2 μL per injection) were injected to 2 mm depth, targeting the septo-diencephalic and septo-striatal subcortex, respectively (supplemental Figure 1B-C). The needle was kept at injection depth for 2 minutes to minimize reflux. Prior to the injections, the transduction efficiencies of AAV2, AAV8, and AAV9 were compared by bilateral deep brain (cauda putamen) injection of vectors encoding green fluorescent protein (GFP). Expression levels were analyzed 7 days after injection under an epifluorescence microscope (supplemental Figure 1D-F).
Immunohistochemistry
Two weeks after the intracranial AAV9-growth factor injection, mice were anesthetized with a lethal dose of xylazine-ketamine and perfused with 1% paraformaldehyde in phosphate-buffered saline (PBS) through the left ventricle. After coronal sectioning at the in-between injection sites, the brains were immersed in 20% sucrose solution at 4°C for 6 hours and frozen in OCT (TissueTek) at −20°C. Coronal sections (40-μm thick ) of the brains were cut in a cryomicrotome (Microm HM 550). The sections were fixed with cold acetone, washed with PBS, and blocked with the mixture of 5% donkey serum, 0.2% bovine serum albumin, 0.3% octyl phenol ethoxylate (Triton-X 100) in PBS.24 The following primary antibodies were used for immunostaining: goat anti-podocalyxin (1:500; R&D Systems), rat anti-platelet endothelial cell adhesion molecule (PECAM-1) (clone MEC 13.3, 1:500; BD Pharmingen), rat anti-platelet-derived growth factor receptor β (PDGFR-β) (clone APB5, 1:500; eBioscience), Cy3-conjugated mouse anti-α-smooth muscle actin (SMA) (clone 1A4, 1:500; Sigma-Aldrich), rat anti-CD45 (clone OX-1, 1:500; BD Pharmingen), rabbit anti-glial fibrillary acidic protein (GFAP) (1:500; Dako), rabbit anti-neurofilament 200 (1:200; Sigma-Aldrich), and rabbit anti-fibrinogen (1:500; Dako). Sections were washed with 0.3% PBS–Triton-X 100 and the unconjugated primary antibodies were detected with the appropriate Alexa 488, 594, or 647 secondary antibody conjugates (Molecular Probes/Invitrogen). All fluorescently labeled samples were mounted with Vectashield containing DAPI (4′,6-diamidino-2-phenylindole) (Vector Laboratories).
Microscopy
Fluorescently labeled samples were analyzed using a compound fluorescence microscope (Zeiss 2; ×10 objective with numerical aperture [NA] 0.30; Carl Zeiss) or a confocal microscope (Zeiss LSM510 Meta, ×10 objective with NA 0.45, ×40 oil objective with NA 1.3; Carl Zeiss) by using multichannel scanning in frame mode, as reported.25 Three-dimensional projections were digitally reconstructed from confocal z-stacks. Colocalization of signals was assessed from single confocal optical sections.
For transmission electron microscopy, intracranial injections of AAV9-mPlGF, and AAV9-mVEGF-B as a control, were carried out as described in “Intracranial subcortical injection of the AAV vectors.” Two weeks after the gene transduction, the animals were sacrificed and the brains were fixed in 1% glutaraldehyde and 4% formaldehyde in 0.1 M phosphate buffer, pH 7.4, postfixed in 1% osmium tetroxide, dehydrated in ethanol, and embedded in Epon LX112 (Ladd Research Industries). Sections (1.0 μm) were stained with toluidine blue for histologic analysis, and 80-nm sections were cut with a Leica Ultracut UCT microtome (Leica Microsystems). At least 5 specimens from each mouse were examined using a JEM 1200 EX (JEOL Ltd.) transmission electron microscope. Images were captured with an Olympus-SIS Morada digital camera.
Vessel morphometry and quantitative analysis
Microvessel area was quantified as the PECAM-1 or podocalyxin-positive area from ×10 confocal micrographs, from at least 3 sections from a minimum 3 injection sites, using the ImageJ software (NIH). Pericyte density was calculated as the PDGFR-β26 positive area from at least 3 sections from a minimum 3 injection sites. CD45-positive cells per injection site were counted under ×10 magnification. At least 3 mice were used per growth factor tested. The contrast of the images was adjusted using PhotoShop software (Adobe). Statistical analysis was performed using 1-way ANOVA from PASW Statistics 19.0. P < .05 was considered statistically significant.
Optical projection tomography
Tissue processing.
Injections of AAV9-VEGF and AAV9-hPlGF were carried out as described in “Intracranial subcortical injection of the AAV vectors,” with the modification of injecting the vectors only to the right side, and performing a sham injection to the left side. Two weeks after the gene transfer, 50 μL of fluorescein isothiocyanate–conjugated Lycopersicon esculentum (tomato) lectin (Vector Laboratories) diluted in 50 μL of PBS, was injected into the femoral vein and allowed to circulate for 5 minutes. Mice were anesthetized with a lethal dose of xylazine-ketamine and perfused with 4% paraformaldehyde in PBS through the left ventricle. Tissue was incubated overnight in 4% paraformaldehyde at 4°C, then embedded in 1% low-melt agarose. The samples were mounted on rotary stages, dehydrated in 75% methanol, and subsequently cleared using a 1:2 mixture of benzyl alcohol and benzyl benzoate.
Data acquisition and visualization.
Optical projection tomography (OPT)27 (Bioptonics OPT 3001) was applied to scan the brains stepwise at 0.9 degree resulting in 400 images of projection data over a complete revolution. Images were taken with the GFP1 (autofluorescence, 425/40 nm exciter, 475 nm emitter) and GFP+ (470/40 nm exciter, 515 nm emitter) filters. Image stacks were reconstructed using NRecon software (version 1.6.3.3; Skyscan), and 3-dimensional volumetric representations were visualized in a Bioptonics viewer (version 2.01; Bioptonics).
Magnetic resonance imaging
Injections of AAV9-VEGF and AAV9-hPlGF, and sham injections, were carried out as described for the OPT in the previous paragraph. Two weeks after the intracranial injections, the T1-weighted RARE (Rapid Acquisition with Relaxation Enhancement) sequence with and without gadolinium (1.0 mmol/kg gadopentetate dimeglumine; Magnevist, Bayer Finland) (repetition time [TR] = 500 milliseconds; echo time [TE] = 10.0 milliseconds; number of averages [NEX] = 7, RARE factor = 4; matrix [MTX] = 256 × 192; field of view [FOV] = 23 × 23 mm), T2-weighted RARE sequence (TR = 2500 milliseconds, TE = 13.3 milliseconds, NEX = 2, RARE factor = 8, MTX = 256 × 256, FOV = 23 × 23 mm) and T2*-weighted FLASH (fast low-angle shot), (TR = 350 milliseconds, TE = 10.0 milliseconds, NEX = 4, MTX = 256 × 128, FOV= 23 × 23 mm) sequences were obtained with a 4.7T MRI scanner (PharmaScan; Bruker BioSpin GmbH). Mice were anesthetized with 1% to 2% isoflurane during magnetic resonance imaging (MRI). Three mice per group were injected, but of the AAV9-VEGF group, an injected mouse died due to intracerebral bleeding prior to the imaging.
Results
VEGF and VEGF-C induce hemangioma formation while PlGF promotes the formation of new microvessels
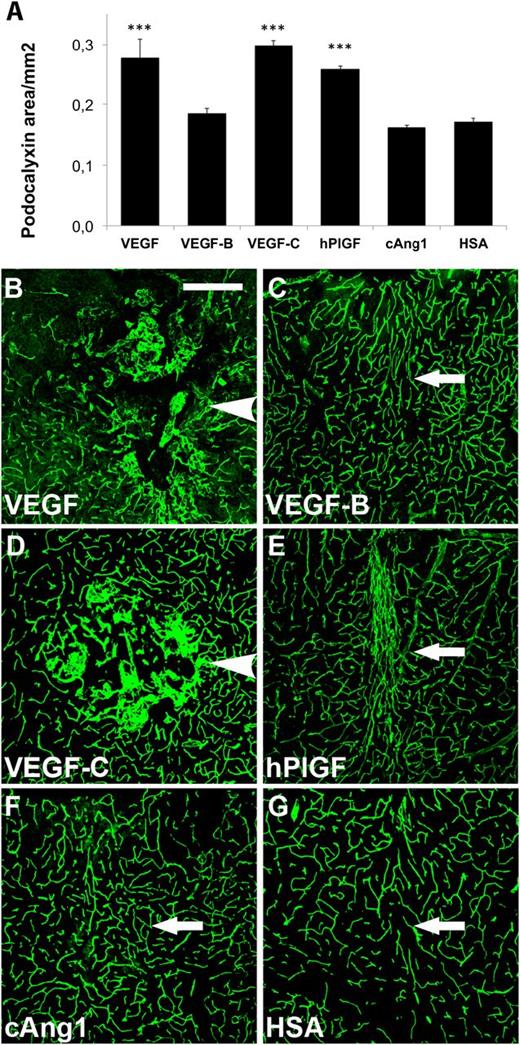
For vascular growth factor expression in the CNS, AAV vectors were delivered to the septo-diencephalic and septo-striatal subcortex by 4 separate injections (supplemental Figure 1A-C). The injection scar could be seen in GFAP immunostaining of tissue sections from the injection area (supplemental Figure 1C). When comparing GFP gene transduction using AAV2, AAV8, and AAV9 vectors, AAV9-GFP gave the most robust expression at 7 days (supplemental Figure 1D-F). We therefore transduced the mouse brain with AAV9s encoding the human vascular growth factors. Two weeks following the injection, we observed that PlGF, VEGF, and VEGF-C22 had stimulated a comparable increase in the vascular area, as quantified by both podocalyxin and PECAM-1 immunofluorescence (Figure 1A; supplemental Figure 2A). VEGF and VEGF-C promoted the formation of hemangioma-like glomeruloid structures (Figure 1B,D; supplemental Figure 2B,D), which were not observed in brains expressing the other factors. In contrast, PlGF promoted the formation of more organized vessels (Figure 1E; supplemental Figure 2E), whereas a 1:1 mixture of the 2 isoforms of VEGF-B (VEGF-B167 and VEGF-B186) did not promote angiogenesis (Figure 1A,C; supplemental Figure 2A,C). The injection itself did not provoke a significant angiogenic response (Figure 1A,G; supplemental Figure 2A,G).
Effects of human vascular growth factor gene transduction on the brain vasculature. Immunostaining of the targeted brain regions 2 weeks after transduction. Quantification of (A) podocalyxin-positive areas. ***P < .001, when compared with HSA control; error bars show SD. (B-G) Examples of podocalyxin staining of (B) VEGF, (C) VEGF-B, (D) VEGF-C, (E) PlGF, (F) cAng1, and (G) HSA transduced brains. (B,D) Arrowheads point to hemangiomas. (C,E-G) Arrows point out needle path. Scale bar, 200 μm.
Effects of human vascular growth factor gene transduction on the brain vasculature. Immunostaining of the targeted brain regions 2 weeks after transduction. Quantification of (A) podocalyxin-positive areas. ***P < .001, when compared with HSA control; error bars show SD. (B-G) Examples of podocalyxin staining of (B) VEGF, (C) VEGF-B, (D) VEGF-C, (E) PlGF, (F) cAng1, and (G) HSA transduced brains. (B,D) Arrowheads point to hemangiomas. (C,E-G) Arrows point out needle path. Scale bar, 200 μm.
In separate experiments, we also tested AAV9s encoding angiopoietins, namely the cartilage oligomeric matrix protein-angiopoietin-1 chimera, (cAng1), an engineered form of the native Ang1 with improved solubility and stability,23 and Ang2 as well as VA1, an in-house engineered chimeric growth factor combining the receptor binding properties of VEGF and Ang1. Although cAng1 did not promote angiogenesis (Figure 1A,F; supplemental Figure 2A,F), its expression seemed to induce dilation of putative postcapillary venules in the brain (supplemental Figures 2F and 3C).28 Also, Ang2 gene transfer failed to promote angiogenesis in the murine CNS (supplemental Figure 3). VA1 increased vessel sprouting significantly, but this did not lead to a significant increase in microvessel density (supplemental Figure 4). In summary, PlGF was the only vascular growth factor that strongly promoted angiogenesis without hemangioma formation. We chose to focus on PlGF in further studies to evaluate its feasibility for proangiogenic therapy in the CNS.
PlGF induces little inflammation in the brain
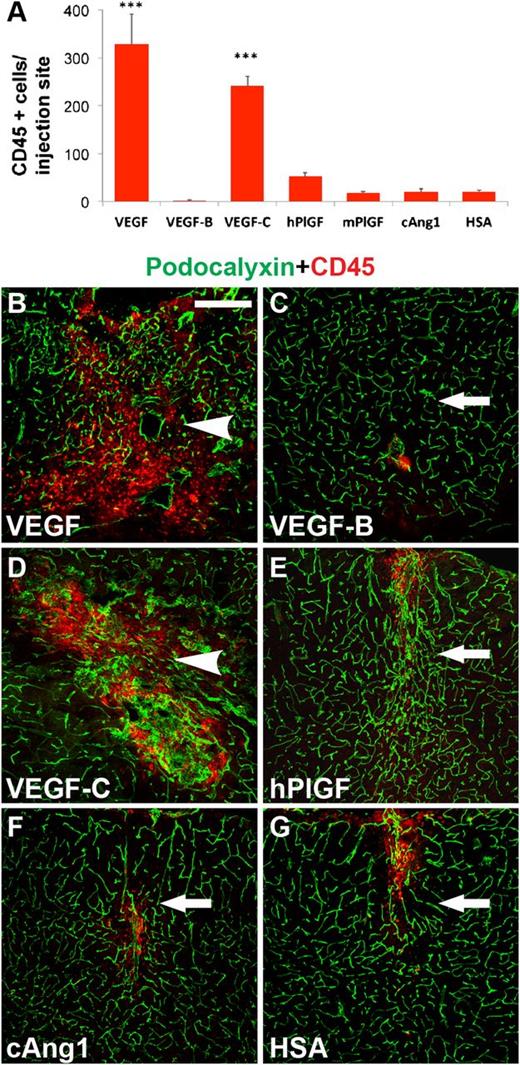
VEGF family growth factors promote unwanted inflammation, which may indirectly contribute to angiogenesis. We therefore next evaluated the extent of inflammatory cell recruitment from the blood circulation upon vascular growth factor expression. Human VEGF and VEGF-C potently recruited CD45-positive leukocytes, and human PlGF showed a trend of increasing inflammatory cell numbers in the injection area when compared with the control (Figure 2A). The pattern of nuclear staining shown in supplemental Figure 5 further demonstrates the increased cell density associated with the leukocyte recruitment. To further test whether the PlGF-evoked inflammation in the treated mice could contribute to the angiogenic effects observed, we measured the PECAM-1–positive area after delivery of AAV9-encoding mouse PlGF. Mouse PlGF showed a trend of recruiting less CD45-positive leukocytes than human PlGF (Figure 2A). However, it was strongly angiogenic (supplemental Figure 6), indicating that the angiogenic effect of human PlGF treatment is not due to inflammation.
Inflammatory response after vascular growth factor expression. (A) Quantitative analysis of inflammation by CD45-positive cell counting per injection site. ***P < .001, when compared with HSA control; error bars show SD. (B-G) Immunostaining for CD45-positive cells (red), vascular counterstaining for podocalyxin (green). Scale bar, 200 μm.
Inflammatory response after vascular growth factor expression. (A) Quantitative analysis of inflammation by CD45-positive cell counting per injection site. ***P < .001, when compared with HSA control; error bars show SD. (B-G) Immunostaining for CD45-positive cells (red), vascular counterstaining for podocalyxin (green). Scale bar, 200 μm.
PlGF-induced microvessels recruit mural cells and arterialize
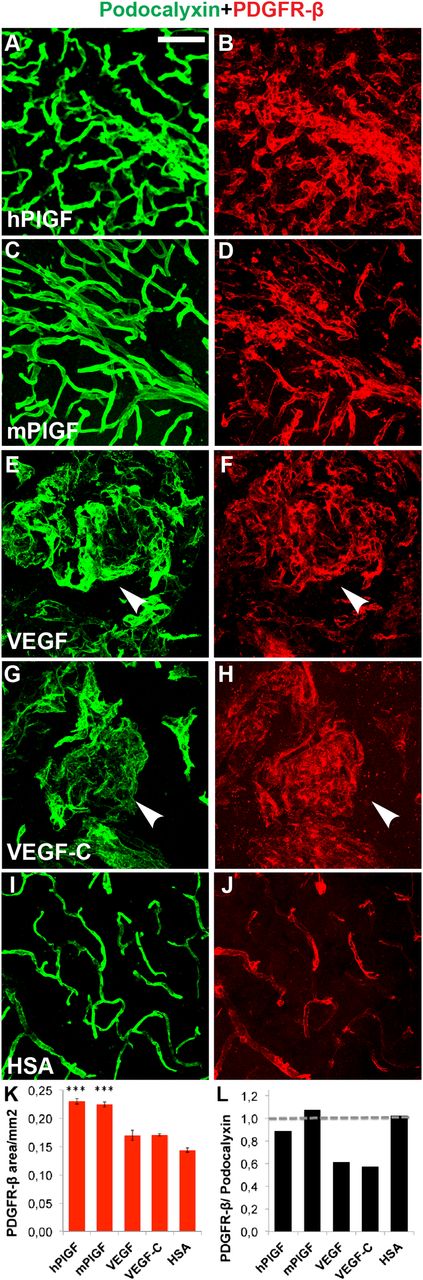
We next wanted to investigate whether the blood vessels formed in response to PlGF displayed hallmarks of mature vessels. We found that the newly formed microvessels were covered almost uniformly by PDGFR-β–positive pericytes26 in samples from PlGF-transduced brains (Figure 3A-D,K-L). In contrast, the neovessels were more sparsely covered by pericytes in brains transduced with VEGF or VEGF-C (Figure 3E-H,K-L).
Immunohistochemical evidence for mural cell recruitment after PlGF treatment. Immunostaining of endothelial cells for (A,C,E,G,I,) podocalyxin (green) and for (B,D,F,H,J) pericytes (PDGFR-β, red). (K-L) Quantitative analysis of (K) PDGFR-β–positive area, and quantitative comparison of (L) podocalyxin vs PDGFR-β–positive areas, with horizontal line at 1, referring to equal area with both stainings. (E-H) Arrowheads point to hemangiomas. ***P < .001 when compared with HSA control; error bars show SD. Scale bar, 50 μm.
Immunohistochemical evidence for mural cell recruitment after PlGF treatment. Immunostaining of endothelial cells for (A,C,E,G,I,) podocalyxin (green) and for (B,D,F,H,J) pericytes (PDGFR-β, red). (K-L) Quantitative analysis of (K) PDGFR-β–positive area, and quantitative comparison of (L) podocalyxin vs PDGFR-β–positive areas, with horizontal line at 1, referring to equal area with both stainings. (E-H) Arrowheads point to hemangiomas. ***P < .001 when compared with HSA control; error bars show SD. Scale bar, 50 μm.
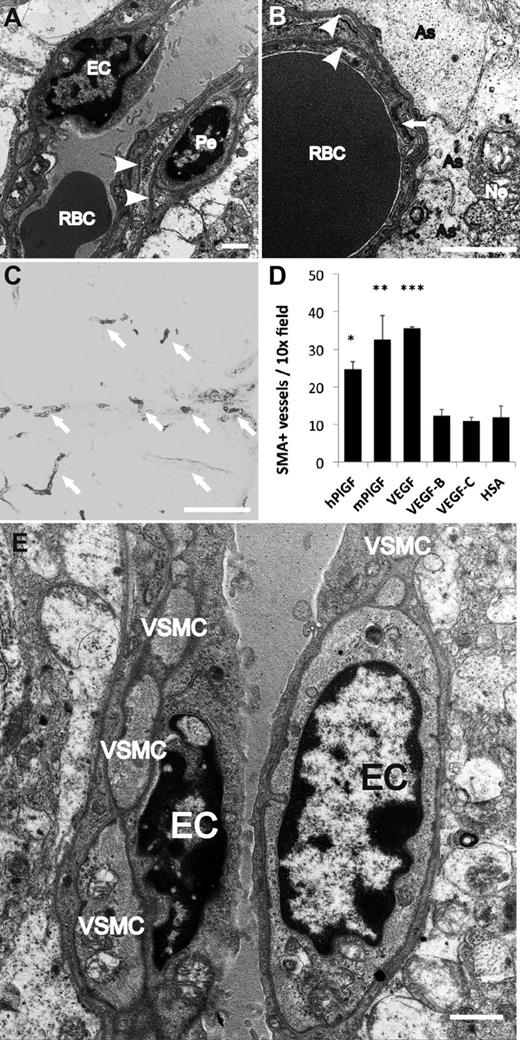
The capillaries formed in response to PlGF displayed mature basal laminae, associations with mural cells and contacts with astrocyte foot processes, as evaluated by transmission electron microscopy (Figure 4A-B). Furthermore, the gene transfer of either mouse or human PlGF significantly increased the number of arterialized microvessels (Figure 4C-D), surrounded by a continuous layer of SMA-positive smooth muscle cells (Figure 4E). VEGF also led to arterialization of some vessels, but these were surrounded by a chaotic accumulation of poorly organized glomeruloid endothelial structures. Interestingly, although VEGF-C greatly increased the vascular area (Figure 1A; supplemental Figure 2A), it failed to increase the number of SMA-positive vessels (Figure 4D).
Electron microscopic evidence for mural cell recruitment after PlGF treatment. (A-B,D) Transmission electron microscopic images of vessels newly formed upon PlGF gene transfer. (A-B) Microvessels are surrounded by 2 layers of (endothelial and pericyte) basal laminae (arrowheads). (B) Capillaries are in intimate contact with the astrocytes (As); arrow points to tight junction between 2 endothelial cells. (C) SMA-positive vessels (arrow) after hPlGF gene transfer, for quantification. (D) Quantification of SMA-positive vessel density from immunostainings. *P < .05; **P < .01; and ***P < .001 when compared with HSA control; error bars show SD. (E) Arterioles are ensheathed in a single layer of VSMC. EC, endothelial cell; Ne, neuron; Pe, pericyte; RBC, red blood cell; VSMC, vascular smooth muscle cell. Scale bars: A-B,E, 1 μm; C, 200 μm.
Electron microscopic evidence for mural cell recruitment after PlGF treatment. (A-B,D) Transmission electron microscopic images of vessels newly formed upon PlGF gene transfer. (A-B) Microvessels are surrounded by 2 layers of (endothelial and pericyte) basal laminae (arrowheads). (B) Capillaries are in intimate contact with the astrocytes (As); arrow points to tight junction between 2 endothelial cells. (C) SMA-positive vessels (arrow) after hPlGF gene transfer, for quantification. (D) Quantification of SMA-positive vessel density from immunostainings. *P < .05; **P < .01; and ***P < .001 when compared with HSA control; error bars show SD. (E) Arterioles are ensheathed in a single layer of VSMC. EC, endothelial cell; Ne, neuron; Pe, pericyte; RBC, red blood cell; VSMC, vascular smooth muscle cell. Scale bars: A-B,E, 1 μm; C, 200 μm.
To further investigate the integrity of the newly formed microvessels upon vascular growth factor treatment, we tested for plasma leakage by staining for extravasated fibrinogen, deposited as fibrin in the perivascular tissue. We observed fibrin outside of the vessels in the VEGF and VEGF-C–treated sections, indicating substantial vessel leakage (supplemental Figure 7). Importantly, no significant plasma leakage was observed after PlGF treatment.
To demonstrate the leakiness of vessels in vivo, we stained blood vessels and macromolecular extravasation by fluorescently labeled lectin29 and visualized the results using OPT (Figure 5). Strong extravasation was observed at the injection site after VEGF gene transfer (Figure 5A,C,E). In contrast, we did not observe plasma leakage in or around the dense network of capillaries formed in response to PlGF treatment (Figure 5B,D,F). Taken together, our results indicate that both mouse and human PlGF induce the formation of mature and arterialized vessels comprising an apparently intact BBB, and with minimal inflammation and vessel leakage.
Evaluation of lectin extravasation with optical projection tomography. Right-sided growth factor injections were performed accompanied by left-sided sham operation. After VEGF gene transfer, major extravasation can be observed at the site of the hemangioma (arrowhead: A,C,E). PlGF gene transfer induced the formation of a dense microcapillary network, without significant extravasation (arrow: B,D,F). Autofluorescence is shown in green; lectin staining of blood vessels and extravasation in blue. ca, caudal; Ro, rostral.
Evaluation of lectin extravasation with optical projection tomography. Right-sided growth factor injections were performed accompanied by left-sided sham operation. After VEGF gene transfer, major extravasation can be observed at the site of the hemangioma (arrowhead: A,C,E). PlGF gene transfer induced the formation of a dense microcapillary network, without significant extravasation (arrow: B,D,F). Autofluorescence is shown in green; lectin staining of blood vessels and extravasation in blue. ca, caudal; Ro, rostral.
PlGF does not promote gliosis or rearrangement of neuronal architecture in the brain
Scar formation in the CNS is referred to as gliosis, characterized by the tissue damage–induced proliferation of astrocytes.30 To test whether the vascular growth factors promote gliosis, we stained the astrocytes by antibodies against GFAP. The neuronal architecture was evaluated by neurofilament staining. PlGF treatment did not induce marked gliosis, and the neuronal architecture was grossly unaffected, when compared with the HSA control (Figure 6A-B,E-G,J). In contrast, the gene transfer of either VEGF or VEGF-C was associated with substantial gliosis, and distortion of neuronal structures (Figure 6C-E,H-J). Taken together, although potent in stimulating blood vessel growth, VEGF and VEGF-C induced hemangioma formation, glial scars, and a mispatterned neuronal architecture, which are undesired side effects of proangiogenic therapy.
Effects of vascular growth factor treatment on the glial and neuronal structures. (A-E) Qualitative analysis of astrocytes by immunostaining for GFAP. Scale bar, 200 μm. (F-J) Qualitative analysis of neuronal structures by immunostaining for neurofilaments. Nuclear staining with DAPI. Scale bar, 50 μm. Arrows point out needle path (A-B,E). Arrowheads point to hemangiomas and its side effects in the (C-D) glial and (H-I) neuronal structures.
Effects of vascular growth factor treatment on the glial and neuronal structures. (A-E) Qualitative analysis of astrocytes by immunostaining for GFAP. Scale bar, 200 μm. (F-J) Qualitative analysis of neuronal structures by immunostaining for neurofilaments. Nuclear staining with DAPI. Scale bar, 50 μm. Arrows point out needle path (A-B,E). Arrowheads point to hemangiomas and its side effects in the (C-D) glial and (H-I) neuronal structures.
PlGF treatment does not distort intracranial anatomy or compromise BBB in vivo
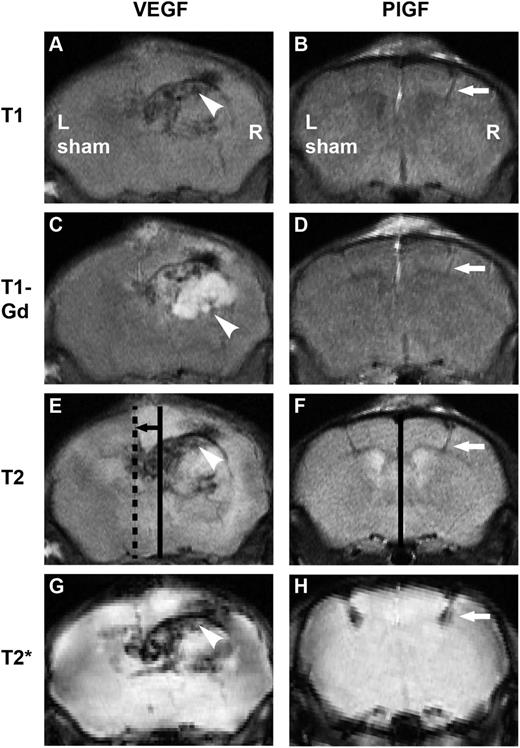
Two weeks after right-sided vascular growth factor and left-sided sham injections, the mice were analyzed by MRI of the brain (Figure 7). VEGF treatment led to an expansive hemangioma and severe, whole-hemispheric edema, causing a major distortion of anatomical structures and a brain midline shift in the T1 sequence. Such severe midline shift and subfalcine herniation is life-threatening. Gadolinium-based contrast agents, routinely used in human diagnostics, give signal enhancement at the sites of BBB disruption.31 Hyperintense enhancement (T1-Gd series, white) revealed the breakdown of the BBB in a strikingly wide region around the VEGF transduction site and the T2 series demonstrated hyperintensity (white) over the majority of the hemisphere, indicating severe edema throughout the hemisphere. The T2* series revealed the extravasation of blood as a hypointense (black) area, showing a large area of microhemorrhages after the VEGF treatment. On the contrary, PlGF transduction did not induce edema or significant BBB breakdown, and the anatomical structures remained intact. Only the injection needle path was detectable as a minor site of extravasation, best visible as hypointesity (black) in the T2* series.
In vivo effects of VEGF and PlGF visualized by MRI. Right-sided growth factor injections were performed accompanied by left-sided sham operation. Incubation time: 2 weeks. (A) After VEGF gene transfer, the macroanatomy of the brain is distorted as observed in T1 images, (C) the BBB is disintegrated from a large area seen as enhancement (white) on T1-Gd series, (E, continuous line: anatomical midline, dashed line: actual midline, black arrow: brain shift) T2 sequence shows major edema (white) with brain shift, and T2* demonstrates hemorrhage (black). Arrowheads point to a hemangioma. Note that after PlGF treatment, (B) the macroanatomy is intact, (D) enhancement is minimal, (F, continuous line: anatomical and actual midline coincide) there is no edema and no brain shift, and (H) minor hemorrhage is only seen at injection path. White arrows point to injection path.
In vivo effects of VEGF and PlGF visualized by MRI. Right-sided growth factor injections were performed accompanied by left-sided sham operation. Incubation time: 2 weeks. (A) After VEGF gene transfer, the macroanatomy of the brain is distorted as observed in T1 images, (C) the BBB is disintegrated from a large area seen as enhancement (white) on T1-Gd series, (E, continuous line: anatomical midline, dashed line: actual midline, black arrow: brain shift) T2 sequence shows major edema (white) with brain shift, and T2* demonstrates hemorrhage (black). Arrowheads point to a hemangioma. Note that after PlGF treatment, (B) the macroanatomy is intact, (D) enhancement is minimal, (F, continuous line: anatomical and actual midline coincide) there is no edema and no brain shift, and (H) minor hemorrhage is only seen at injection path. White arrows point to injection path.
PlGF induces VEGFR-1 but not VEGFR-2 signaling in mouse brain
To analyze the specificity of PlGF-induced VEGFR signaling in the CNS, we first compared the relative expression levels of VEGFR-1 and VEGFR-2 in the brain, kidney, lung, and heart. Immunoblotting showed similar levels of VEGFR-1 in the brain, kidney, and heart, and somewhat higher levels in the lung, whereas VEGFR-2 protein levels were significantly lower in the brain than in the other tissues analyzed (supplemental Figure 8A). In the brain, PlGF induced robust VEGFR-1 phosphorylation, and VEGF activated VEGFR-2 but not VEGFR-1 (supplemental Figure 8B).
Discussion
We carried out a systematic evaluation of vascular growth factors for their angiogenic potential and for their shortcomings in the murine CNS. We identified PlGF as an effective and apparently safe angiogenic growth factor. PlGF gene transfer increased significantly the density of mature microvessels that were covered by mural cells and a basement membrane, and surrounded by astrocyte foot processes, indicating an intact BBB. These findings are in line with our MRI data from living mice. In addition, PlGF stimulated arteriogenesis, as evidenced by an increased abundance of blood vessels ensheathed by a layer of smooth muscle cells.
VEGF and VEGF-C gene transfers promoted vascular leakage, hemangiomas, and robust reactive gliosis. Reactive gliosis induced by VEGF-C was reported by Thomas and colleagues,32 even upon AAV2-mediated gene transfer, which results in low transgene expression in the brain. The newly formed vessels generated in response to PlGF were nonleaky and gliosis was not observed. VEGF treatment induced severe, panhemispheric edema, demonstrating that the effects and side effects of treatment may involve an area far larger than the region transduced.
The favorable angiogenic effect observed only after PlGF treatment in our study may reflect the unique VEGFR-1/VEGFR-2 receptor expression and specific activation pattern that we observed in the brain. The angiogenic effect of PlGF is considered to result directly from activation of VEGFR-1 in endothelial cells, as well as indirectly via activation of VEGFR-1 signaling in monocytes that produce angiogenic factors and proteolytic enzymes, facilitating angiogenesis and arterialization of microvessels.33-35 Monocyte recruitment is thought to be critical for the arterialization of microvessels,34 which was also observed in our experiments. However, mouse PlGF did not promote leukocyte recruitment over baseline (HSA) in the CNS, unlike in skeletal muscle,34 suggesting that it can stimulate leukocyte functions that promote angiogenesis and arteriogenesis.
In addition to PlGF, both VEGF and VEGF-B bind to VEGFR-1. However, the level of VEGFR-1 activation differs upon VEGF vs PlGF treatment. Although VEGF-B binds to VEGFR-1 and can stimulate its phosphorylation in endothelial cells, its angiogenic effect is weak and, intriguingly, largely restricted to the heart.5 We did not observe angiogenesis in the CNS in response to VEGF-B stimulation. However, unlike VEGF or VEGF-C, PlGF did not activate VEGFR-2, which potently promotes the disassembly of endothelial cell-cell junctions and plasma leakage. The pathological angiogenic response obtained by VEGF and VEGF-C stimulation may in part depend on the fact that prolonged ligand stimulation of VEGFR-2 leads to its increased expression in cerebral vessels.36 Besides binding to VEGFR-1, the heparin-binding form of PlGF (PlGF-2), used in our study, also binds the VEGF coreceptors neuropilin-1 and neuropilin-2. Neuropilin-1 is necessary for arterial differentiation during embryonic development,37 suggesting that it contributes to the arteriogenesis observed in our model system. Furthermore, neuropilin-1 signaling promotes sprouting and induction of pericyte-associated functional microvessels.38 Neuropilins are also expressed in a subpopulation of Tie2+ macrophages that have been shown to act as cellular chaperones in vascular morphogenesis,39 which may contribute to the effects observed. Although VEGFRs and neuropilins are also expressed in neuronal cells and contribute to several processes including axon pathfinding during embryogenesis,40,41 effects on neuronal patterning were not evident in response to PlGF or VEGF-B in our study. We did not observe increased microvessel density in the CNS after treatment with cAng1, Ang2, or VA1, which is why they were not chosen for further analysis.
Previous attempts to revascularize the CNS in model organisms have focused on VEGF, and found that postischemic intravenous delivery of VEGF tended to worsen disease outcome by promoting hemorrhage and by increasing plasma leakage through the BBB.42,43 In contrast, VEGF gene transfer via naked plasmids, now an obsolete technology, to tissues adjacent to brain parenchyma was marginally beneficial,44 but possible adverse effects were not evaluated in great detail. Yet, previous studies have not provided a systematic comparison of multiple candidate vascular growth factors in the CNS.
Indirect EC-IC bypass procedures, that is, bringing highly vascularized tissue into direct contact with the hypoperfused brain without performing direct anastomosis, lead to sufficient revascularization of the hypoperfused brain in the pediatric cases of moyamoya disease.11 However, the indirect EC-IC procedure is ineffective in adults.45 An interesting approach would be to use growth factor gene transfer to augment indirect EC-IC bypass procedures. For example, angiogenic growth factors could be used to bridge the highly vascularized scalp and the ischemic brain via multiple drilled, so-called burr holes in the skull.46 Growth factor–enhanced multiple burr hole EC-IC bypass could provide a technically simple method for revascularization of the CNS. Furthermore, the revascularization would be gradual, which may help avoid major complications such as the hyperperfusion syndrome.14
Our results identify PlGF as a candidate for therapeutic revascularization of the CNS, hence paving the way for further translational studies involving models of brain ischemia. These findings should lead to the development of new clinical approaches to treat acute and chronic cerebral hypoperfusion, including the combination of growth factor therapy with vascular bypass surgeries, which could ultimately benefit patients suffering from vascular dementia, stroke, and other neurovascular diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jere Paavola for contributing to the project ideas. Dr Fang Zhao’s expert help with interpreting electron microscopic images is greatly appreciated. They kindly thank Drs Gou Young Koh, Peter Carmeliet, and Jean-Leon Thomas for providing the cAng1 cDNA, the mPlGF-2 cDNA, and antibodies for our experiments. They are grateful for the excellent technical assistance of Tapio Tainola, Katja Salo, Kirsi Lintula, Niina Ihalainen, and Tanja Laakkonen. The Biomedicum Molecular Imaging Unit and the Advanced Microscopy Unit are acknowledged for microscopy services. The AAV Gene Transfer and Cell Therapy Core Facility is thanked for help with virus production as are the personnel of the Meilahti Experimental Animal Center for expert animal husbandry.
This work was supported by grants from the Academy of Finland (K.A.), the Leducq Foundation (K.A.), the European Research Council (ERC-2010-AdG-268804-TX-FACTORS, PITN-GA-2012-317250-VESSEL), The Finnish Foundation for Cardiovascular Research (K.A.), The Finnish Medical Foundation (E.I.G.), the Orion-Farmos Research Foundation (E.I.G., T. Tammela), the Maire Taponen Foundation (E.I.G.), the Oskar Öflund Foundation (E.I.G.), Biomedicum Foundation (E.I.G.), the Finnish Society of Angiology (E.I.G.), and the Sigrid Juselius Foundation (T. Tammela).
The sponsors of this study are public organizations that support science in general. They had no role in gathering, analyzing, or interpreting the data.
Authorship
Contribution: E.I.G., T. Tammela, A.A., T. Tatlisumak, S.M., P.H., G.Z., and V.-M.L. performed experiments; E.I.G., T. Tammela, and A.A. analyzed results; E.I.G. and A.A. made the figures; and E.I.G., T. Tammela, M.N., J.H., and K.A. designed the research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Petri Honkanen died on March 14, 2013.
Correspondence: Kari Alitalo, Wihuri Research Institute, Biomedicum Helsinki, University of Helsinki, P.O.B. 63 (Haartmaninkatu 8), 00014 Helsinki, Finland; e-mail: Kari.Alitalo@Helsinki.Fi.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal