In this issue of Blood, Massa et al demonstrated that tumor-tropic bacteria engineered to express specific cancer-related antibodies can recognize and specifically destroy the malignant cells by infecting them with toxic cargo. The findings provide an essential step in establishing the novel approach of enlisting engineered bacteria that speak cancer’s language to outsmart and defeat cancer.1
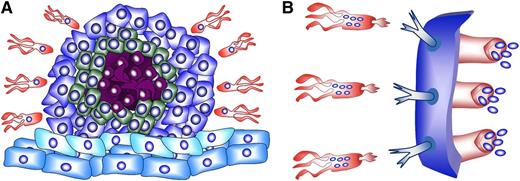
Schematic illustration of the new strategy to fight cancer using engineered Trojan-horse bacteria. (A) Illustration of the migration of cargo-carrying tumor-tropic bacteria toward a tumor. The outer cells (dark blue) are proliferating cells. The green cells represent dormant cancer stem cells (CSCs). Note that in real tumors the fraction of this subpopulation of cells is very low, and they can be widely scattered. The red cells are necrotic cells, and the light blue cells are stromal cells (see Ben-Jacob et al4 for more details). (B) Illustration of cargo-carrying Trojan-horse bacteria engineered to express antibodies targeting the tumor-specific antigens. This recognition enables the bacteria to enter the cells and inject the antitumor cargo.1
Schematic illustration of the new strategy to fight cancer using engineered Trojan-horse bacteria. (A) Illustration of the migration of cargo-carrying tumor-tropic bacteria toward a tumor. The outer cells (dark blue) are proliferating cells. The green cells represent dormant cancer stem cells (CSCs). Note that in real tumors the fraction of this subpopulation of cells is very low, and they can be widely scattered. The red cells are necrotic cells, and the light blue cells are stromal cells (see Ben-Jacob et al4 for more details). (B) Illustration of cargo-carrying Trojan-horse bacteria engineered to express antibodies targeting the tumor-specific antigens. This recognition enables the bacteria to enter the cells and inject the antitumor cargo.1
Despite much-improved knowledge of cancer biology, cancer continues to elude us and remains undefeated. Its most alarming aspects, namely, metastasis, relapse, and multiple drug resistance, are still inadequately understood and clinically insuperable.2-4 It has become widely acknowledged that creative new ideas and out-of-the-box approaches to fight cancer ought to be explored. A promising new strategy is to recruit anaerobic bacteria that preferentially colonize and proliferate in the hypoxic environment of tumors.
Why bacteria and cancer? Increasing numbers of recent studies have revealed parallels between bacteria and cancer (eg, rapid proliferation, rapid development of drug resistance, and high phenotypic variability).4 It has been conjectured that cancer shares so many features in common with bacteria because it represents an atavistic form of life, which ensued following a breakdown of regulatory pathways that evolved upon the transition from prokaryotes to eukaryotes.5 This breakdown unlocked “ancient toolkits,” and thus cancer cells resort to more fundamental strategies that have been developed by bacteria through the course of evolution. The underlying foundations of the bacteria-cancer parallels provide the rationale as to why wisely engineered bacteria can provide successful smart troops to fight cancer.
It has been shown that tumor-tropic bacteria can be recruited to assist the immune system to fight cancer.6 Recent studies have demonstrated that the same capabilities that these smart organisms employ to trick the immune system can be turned to outsmart cancer. Injected Salmonella can cause melanoma cells to form gap junctions with adjunct immune dendritic cells.7 Consequently, the dendritic cells use peptides transferred from the cancer cells to “teach” T cells to recognize and kill the tumor cells at the primary site and prevent metastasis formation.
The current studies of Massa et al1 provide a crucial leap toward establishing this promising approach. The authors engineered nonvirulent tumor-tropic Salmonella typhimurium bacteria to selectively express CD20-targeting antibodies and demonstrated that the bacteria selectively infect CD20+ lymphoma cells both in vitro and in vivo. Moreover, having been engineered to carry antitumor cargo (the herpes simplex virus thymidine kinase), the cancer-invading bacteria selectively transferred the harmful cargo and killed the cancer cells (see figure), so much so that the bacteria could successfully fight human lymphoma xenografts in mice lacking functional immune systems while protecting them from the dangerous virus. This vital proof-of-concept study kindles hope that the same strategy can be employed to target other types of cancer and selectively deliver and inject antitumor compounds directly into the cancer cells. Therefore, the new strategy paves the way to administer a range of potent drugs, which are not used currently because of their high toxicity to the body.
The new strategy also provides hope for successfully treating CSCs by engineering bacteria to express antibodies targeting specific markers of these cells. Although there is an ongoing debate regarding the terminology and the exact nature of CSCs, it is commonly agreed that tumors have small subpopulations of special tumorigenic cells with the ability of renewal and differentiation into a variety of cell types and cause tumor relapse.8 These subpopulations pose a high risk because they are not detected by standard imaging methods, and since they can become dormant and thus successfully evade ordinary cancer treatments (chemotherapy, radiation therapy, and targeted/biological therapy). Development of specific therapies targeted at these transitional CSCs holds hope for improvement of survival and quality of life of cancer patients, especially for sufferers of metastatic disease. The advantage of the bacteria-based strategy is that the Trojan-horse bacteria engineered to express markers of both the tumor cells and the CSCs are expected to be able to find the dormant CSCs hiding within the tumor and attack them with newly discovered compounds effective against dormant cells.
An intriguing example of such a compound is salinomycin which is secreted by the Streptomyces albus bacteria.9 This widely used agricultural agent has recently been shown to be highly potent against CSCs. As promising as it is, salinomycin cannot be used currently because of its high general toxicity. The Trojan-horse bacteria may be able to deliver the compound to kill the CSCs while protecting the rest of the body from the drug toxicity.
To conclude, building on the results reported by Massa et al,1 we can envision recruiting engineered Trojan-horse bacteria to explore the body and find cancer cells. The engineered bacteria can then kill the cancer cells directly or expose them to the immune system and to other recruited bacteria engineered to kill them by acting together with the immune system. For example, it is now understood that most tumors at advanced stages are composed of several subclones. We can foresee the engineering of several Trojan-horse bacteria acting together, each able to better identify and target a specific clone. We may be seeing here the dawning of a new era of biological cyberwarfare on cancer, in which we will enlist bacteria to fight with the immune system and defeat cancer with minimal side effects to the patients.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal