Abstract
Umbilical cord blood is an alternative hematopoietic stem cell source for patients with hematologic diseases who can be cured by allogeneic hematopoietic cell transplantation. Initially, umbilical cord blood transplantation was limited to children, given the low cell dose infused. Both related and unrelated cord blood transplants have been performed with high rates of success for a variety of hematologic disorders and metabolic storage diseases in the pediatric setting. The results for adult umbilical cord blood transplantation have improved, with greater emphasis on cord blood units of sufficient cell dose and human leukocyte antigen match and with the use of double umbilical cord blood units and improved supportive care techniques. Cord blood expansion trials have recently shown improvement in time to engraftment. Umbilical cord blood is being compared with other graft sources in both retrospective and prospective trials. The growth of the field over the last 25 years and the plans for future exploration are discussed.
Introduction
This year marks the 25th anniversary of the first umbilical cord blood (UCB) transplantation (UCBT) performed in France in a child with Fanconi Anemia (FA). Over the last 25 years, the field of UCB banking and transplantation has grown exponentially. Over 600 000 UCB units have been stored for transplantation worldwide, and >30 000 UCBTs have been performed. UCB serves as an alternative stem cell source; only 30% of patients who require an allograft will have a human leukocyte antigen (HLA)-matched sibling donor. Despite >20 million adult volunteer donors in the National Marrow Donor Program and affiliated registries,1 many patients, particularly patients of diverse racial/ethnic backgrounds, will not have a suitably matched, unrelated volunteer donor identified in the required time period. UCB has extended access to transplantation, especially to patients of racial and ethnic minorities,2 and is rapidly available. In this review, the scientific basis of UCBT is discussed, the historic first UCBT is revisited, and recent pediatric and adult UCBT outcome data are presented. Strategies for future improvement include: utilization of UCB expansion, ex vivo and in vivo homing techniques, in vivo nurturing procedures, selection of the optimal UCB unit, and enhancement of immune recovery.
Scientific basis of cord blood transplantation
Using UCB as a source of transplantable hematopoietic stem (HSC) and progenitor (HPC) cells was suggested by Hal Broxmeyer in a private meeting with the late Edward A. Boyse and Judith Bard in 1982. Boyse felt discarding the UCB after the birth of a baby was wasteful. Broxmeyer believed the discarded UCB might be better used, not as Boyse suggested as a source of mature cells for transfusion, but as a source of transplantable HSC and HPC. This meeting led to the formation of Biocyte Corporation, a UCB company founded by Boyse, Bard, Lewis Thomas, Broxmeyer, Harvey Cantor, Rodman Rockefeller, and George Strong. After many meetings to discuss the concepts, possibilities, and ethical considerations of UCBTs, Biocyte funded Broxmeyer, at the Indiana University School of Medicine (IUSM), with a 2-year grant to study the biology and cryopreservation of UCB cells. UCB cells for study were obtained at the IUSM and later in larger numbers from Gordon Douglas at the New York University Medical Center. These studies established the possibility of using UCB as a transplantable source of HSCs and HPCs, which then led to the first UCBT and subsequent UCBTs.3-7 The scientific and clinical papers were both published in 1989, as the investigators waited years after obtaining the laboratory data until it was clear that the UCB engrafted in the first UCBT recipient before publishing the scientific paper.
The scientific findings established the framework for the first UCBT. HPCs in UCB had an extensive proliferative capacity that exceeded that of bone marrow (BM) HPC, and numbers of HPC in single UCB collections were within the range of HPC numbers associated with successful BM transplant (BMT).3 In fact, the eureka moment for Broxmeyer came when it was realized that efforts to separate UCB cells by gravity or into a low-density mononuclear cell fraction led to unacceptable losses of HPC and that unseparated UCB cells yielded many more HPCs than after cell separation.3 In addition to findings that UCB could be left for days at room temperature without significant loss of functional HPCs and that the UCB could be sent by overnight-express mail from New York to the Broxmeyer laboratory where these cells could be cryopreserved and later thawed with efficient recovery of HPC, it was realized that there were many more HPCs present in a single collection of UCB than previously appreciated. These findings, plus a pre-term mouse blood engraftment study in a lethally irradiated congenic mouse model, gave the investigators confidence to use UCB for a clinical HSC transplant (HCT).8 The first clinical UCBT trials were done with unseparated cells. Sibling donor UCB used for the first 5 human UCBTs came from the first proof-of-principle UCB bank established in the Broxmeyer laboratory.
UCB can be stored cryopreserved for >20 years with efficient recovery of HSCs and HPCs,9 and HSCs from UCB have an extensive engrafting capacity that exceeds that of BM in recipient immune cell-deficient mice, an assay not available at the time of these initial biological studies on UCB.9-11 Human HSC assays at that time were in vitro surrogate assays and did not detect long-term marrow-repopulating HSCs. Studies reported since that initial laboratory paper established the extensive proliferative and secondary transplant capacities of UCB compared with those of cells from BM.3,8-16
Once it was decided to attempt a UCBT, FA was selected as a first disease to treat, because HLA-matched sibling donor BMT was a treatment option for FA and in families who had affected children with FA, there was the possibility of using BM from nonaffected siblings who were an HLA match for the affected child. The first UCBT was done in Paris, as Gluckman had the best clinical results at that time using BMT to treat patients with FA.17-19 The UCB cells were infused into the conditioned recipient without separation or washing to ensure little or no loss of these precious cells. Later studies demonstrated volume-reduced UCB units were acceptable for UCBT.
The first cord blood transplant
The first UCBT, performed in October 1988, was made possible by an international collaboration between Arleen Auerbach from the Rockefeller University in New York, who described a method of prenatal diagnosis in FA,17 Broxmeyer from IUSM, and Gluckman from the Hospital Saint Louis in Paris, who demonstrated that the in vivo hypersensitivity of FA cells caused increased toxicity in the pretransplant conditioning regimen used in aplastic anemia18 and who was the first to use modified, attenuated, dose conditioning in these patients to improve short- and long-term survival.19 UCB was collected by Dr. Douglas at the birth of a female baby, found by prenatal diagnosis using cultured amniotic fluid cells to be unaffected with FA and HLA-identical to a brother with FA, and the UCB was cryopreserved at the IUSM.4 The physicians and the human subjects institutional review boards of the involved centers felt that the availability of UCB blood obviated the need for BM aspiration from the infant sibling, although the infant sibling was available for BM donation if necessary. Prior to transplant, the French National Ethics Committee gave authorization to perform the UCBT, as the young sibling donor would not be put at risk of a general anesthesia and the UCBT procedure, conditioning regimen, and supportive care were well validated by published results. The UCBT was considered an urgent life-saving treatment. UCB had never been used before in humans, but cryopreserved BM cells had been proven safe and effective. UCB was considered a waste product, although now regulated as a therapeutic product in most countries.
The recipient was a 5-year-old patient with severe aplastic anemia due to FA, whose condition necessitated an urgent HCT.4 The patient was conditioned by a procedure developed specifically for the treatment of patients with FA using low-dose cyclophosphamide (20 mg/kg instead of 200 mg/kg) and 5 Gy total lymphoid irradiation. The frozen cells were hand-delivered from Indiana to Paris in a dry shipper that maintained the temperature at −175°C. Cells were thawed without further processing on day 0. Aliquots of these frozen cells were pretested for viability and HPCs before shipping and also after thawing. Results were similar to the counts before freezing. The first signs of engraftment occurred on d 22, with subsequent complete hematological reconstitution and donor chimerism. The patient had no graft-versus-host disease (GVHD) and is currently healthy with complete long-term hematological and immunological donor reconstitution 25 years after UCBT.
Pediatric UCBT
This success opened the way to a new field in allogeneic HCT, as it showed that: (1) a single UCB contained enough HSCs to definitively reconstitute the host lympho-hematopoietic compartment; (2) a UCB unit could be collected at birth without any harm to the newborn infant; and (3) UCB HSCs could be cryopreserved and transplanted into a myeloablated host after thawing without losing their repopulating capacity. The main practical advantages of using UCB are the relative ease of procurement, the absence of risk for mothers and donors, the reduced likelihood of transmitting infections, and the ability to store fully tested and HLA-typed UCB in the frozen state, available for immediate use.
Following this first successful transplant, UCB banks were established in order to collect and cryopreserve UCB for related and unrelated use. In Europe, the largest banks were in Dusseldorf, Milan, London, and Paris. In the US, the New York Blood Center, under the direction of Pablo Rubinstein, established the biggest unrelated UCB bank and reported the first largest cohort of unrelated UCBTs.20 For many years, most UCBTs were given to children, because it was thought that the low number of cells in a single UCB would not be sufficient to engraft an adult. Today, in the Eurocord registry, related UCBTs represent 8% of a total of 9419 UCBTs performed with European UCB units. Related UCBTs are not often performed, because most of the patients do not have a pregnant mother and because of the limited number of directed UCB banks for family use.21-23
In 2000, in a Center for International Blood and Marrow Transplantation Research (CIBMTR)-Eurocord study comparing pediatric BMTs and UCBTs from HLA-identical siblings, UCBT was associated with delayed granulocyte and platelet engraftment, reduced acute and chronic GVHD, but similar survival. This was the first analysis that demonstrated, unambiguously, that GVHD was reduced when UCB cells were used instead of BM.22
Results of related cord blood transplants for children with malignancies have been summarized by Eurocord.24 In 147 patients, most with acute leukemia, the cumulative incidence of neutrophil recovery was 90%, and the incidences of acute and chronic GVHD were 12% and 10% at 2 years, respectively. At 5 years, the cumulative incidences of nonrelapse mortality and relapse were 9% and 47%, respectively, and the probability of disease-free survival (DFS) was 44%. Cell dose and disease status were important factors for outcomes after related UCBT.
The first unrelated UCBTs in children were reported by Joanne Kurtzberg et al25 in 25 children with a variety of malignant and nonmalignant diseases. The 100-day overall survival (OS) was 64%, demonstrating the feasibility of unrelated mismatched UCBTs. Since then, registries and single center reports confirmed favorable outcomes in children with malignant and nonmalignant hematological diseases.26-30 Furthermore, a comparison of unrelated HLA-mismatched UCBTs to matched unrelated donor (MUD) transplants showed that UCBT resulted in a delayed engraftment, less acute and chronic GVHD, and similar relapse rate, OS, and leukemia-free survival (LFS) compared with MUD BM or peripheral blood stem cell transplant.31 Further, when compared with haplo-related transplants for Severe Combined Immunodeficiency Syndrome, DFS was identical, but immune reconstitution and chimerism were better after UCBT.32
In children with malignant diseases, 2 studies compared the outcome of unrelated UCBT and BMT. Eurocord compared the outcomes of matched unrelated BMT (HLA 6 of 6) either unmanipulated or T-depleted to mismatched UCBT.31 After UCBT, engraftment was delayed, and GVHD was similarly reduced to T-cell–depleted BMT; relapse and LFS were similar. Eapen et al33 for the CIBMTR compared outcomes of 503 children with acute leukemia receiving unrelated mismatched UCBT to 282 children receiving a MUD HCT. HLA allele-mismatched BM recipients had more acute and chronic GVHD. Importantly, LFS was not statistically different between BM and 1 or 2 HLA-disparate UCBTs; HLA-matched UCBT recipients had better outcomes compared with HLA allele-matched BM recipients. However, increased transplant-related mortality (TRM) was observed in children transplanted with a low-UCB cell dose (<3 × 107 total nucleated cells [TNCs]/kg) and 1 HLA-disparate UCB graft or in children given a 2 HLA-disparate UCBT independently of the cell dose infused.
A meta-analysis of studies of UCBT and UBMT in children found that the incidence of chronic GVHD was lower with UCBTs, but the incidence of grade III–IV acute GVHD did not differ.34 There was no difference in 2-year OS in children. In children with nonmalignant diseases transplanted with HLA-mismatched UCBT, the results showed a survival rate of 40%. This high failure rate was due to increased risk of rejection and delayed immune recovery. Factors associated with better survival were a higher TNC/kg of <5 × 107 and better HLA matching.35,36 A preliminary analysis of a randomized study comparing double and single UCBT in children did not show any survival advantage to using double UCBT.37
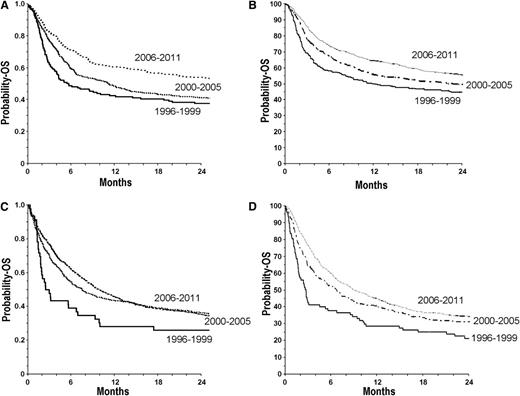
Progress has been made over the years in patient selection, modification of the conditioning regimen, and better choice of the UCB according to cell dose and HLA typing, factors contributing to the improvement of pediatric UCBT results and an increased demand for high-quality UCB units (Figure 1). In the future, new indications for UCBT might be developed in nonhematologic diseases, such as autoimmune diseases or degenerative diseases. Increasing the quality and diversity of UCB units may help to improve results for black patients, whose survival has been inferior to white patients in a large registry study.38
OS at 2 y after UCBT for patients with acute myeloid leukemia, acute lymphoid leukemia, and myelodysplasia in Europe and North America. (A) Children (≤16 years) from Europe: UCBT period 1996-1999 (n = 142), OS: 37 ± 4%; 2000-2005 (n = 441), OS: 41 ± 2%; 2006-2011 (n = 749), OS: 54 ± 2%. (B) Children (≤16 y) from North America: UCBT period 1996-1999 (n = 276), OS: 45 ± 6%; 2000-2005 (n = 843), OS: 50 ± 3%; 2006-2011 (n = 993), OS: 56 ± 6%. (C) Adults from Europe: UCBT period 1996-1999 (n = 46), OS: 26 ± 6%; 2000-2005 (n = 339), OS: 37 ± 3%; 2006-2011 (n = 1595), OS: 36 ± 2%. (D) Adults from North America: UCBT period 1996-1999 (n = 87), OS: 22 ± 8%; 2000-2005 (n = 359), OS: 31 ± 4%; 2006-2011 (n = 1210), OS: 34 ± 3%.
OS at 2 y after UCBT for patients with acute myeloid leukemia, acute lymphoid leukemia, and myelodysplasia in Europe and North America. (A) Children (≤16 years) from Europe: UCBT period 1996-1999 (n = 142), OS: 37 ± 4%; 2000-2005 (n = 441), OS: 41 ± 2%; 2006-2011 (n = 749), OS: 54 ± 2%. (B) Children (≤16 y) from North America: UCBT period 1996-1999 (n = 276), OS: 45 ± 6%; 2000-2005 (n = 843), OS: 50 ± 3%; 2006-2011 (n = 993), OS: 56 ± 6%. (C) Adults from Europe: UCBT period 1996-1999 (n = 46), OS: 26 ± 6%; 2000-2005 (n = 339), OS: 37 ± 3%; 2006-2011 (n = 1595), OS: 36 ± 2%. (D) Adults from North America: UCBT period 1996-1999 (n = 87), OS: 22 ± 8%; 2000-2005 (n = 359), OS: 31 ± 4%; 2006-2011 (n = 1210), OS: 34 ± 3%.
Adult UCBT
High-dose myeloablative single unit UCBT
After promising results in children, the initial UCBT experience with adults was poor, with 40% of patients dying before day 100.39 Advancements in adult single UCBT followed over the next several years, with improved patient selection, better supportive care, and the realization that a higher infused cell dose was associated with superior survival (Figure 1). A multicenter study of 514 patients reported a 1-year survival of 37%, with older age and advanced disease predictors for worse survival.40 Robin et al41 and Sanz et al42 have reported encouraging results in myelodysplasia and early-stage hematologic malignancies with DFS of 30% and 46%, respectively. A Japanese group demonstrated a remarkable DFS of 63% after myeloablative single unit UCBT,43 which may be related to the smaller size of patients, genetic homogeneity, or stricter patient selection.
Enhancing the efficacy of UCBT
Double cord blood transplantation
Improved survival in adult UCBTs followed the observation that cell dose was critical for engraftment and survival, leading to studies on double UCBT. Double UCBT became especially popular in the United States due to the relatively higher weight of the population. In addition, the use of nonmyeloablative or reduced intensity conditioning (RIC), pioneered in related donor and MUD transplantation, allowed older patients and those with comorbidities to be transplanted safely.44,45 Both of these approaches were combined, initially by the Minnesota group, by using a RIC regimen of fludarabine, cyclophosphamide, and low-dose, total-body radiation and by infusing 2 partially matched UCB units.46 Numerous other studies confirmed these observations, reporting a DFS of 30% to 50% after RIC double UCBT in adults.47-49
Recent data have questioned the benefit of double as opposed to single UCBT. In adults, Eurocord retrospective analysis reported improved DFS for leukemia patients in CR1 receiving a double as opposed to single UCBT but no advantage for patients in CR 2.50 These data suggest that single UCBTs may be appropriate for most children; the data for adults requires further investigation.51
HLA and selection of the best UCB unit
Advances in UCB unit selection have also led to better UCBT outcomes.52 The Eurocord group has reported an improvement in DFS from 23% prior to 2000 to 38% in recent years in single, myeloablative UCBTs.53 In an analysis of 1061 single adult and pediatric UCBT recipients for leukemia or myelodysplasia, the lowest TRM was seen in recipients of 6/6 HLA-A,-B antigen, -DRB1 allele-matched units, regardless of cell dose.54 A sliding scale interaction was seen in recipients of mismatched units such that the greater the mismatch, the greater the requirement for TNC dose. Units that were 4/6 HLA-matched to the recipient required a TNC >5.0 × 107/kg to achieve a similar TRM as 5/6 units with a TNC >2.5 × 107/kg. The presence of HLA antibodies against the UCB units has been shown to be a negative prognostic factor for both single and double UCBT.55-57 Two recent studies demonstrated a survival advantage (5-year OS of 55% vs 38%) to choosing UCB units in which maternal typing of the UCB donor showed a match of the noninherited maternal allele to the patient.58,59 Preliminary studies suggest that matching the UCB unit and patient at HLA-C may be beneficial.60,61 Finally, the impact of donor killer-immunoglobulin receptor ligand matching is unclear.62,63
Comparison of graft sources: what is the optimal graft source for adults without a matched sibling donor?
There have been no completed randomized prospective studies to determine the optimal graft source for adults.64 Multiple retrospective studies have indicated comparable survival between both single (Table 1) and double (Table 2) UCB grafts with that of adult donors. Eapen and colleagues65 compared results of 165 single UCBT adult patients, 888 MUD peripheral blood stem cell patients, and 472 MUD BM patients. TRM was higher for the UCBT patients, but acute and chronic GVHD were lower. DFS was comparable among UCBT, fully matched MUD, and mismatched MUD patients, with disease status the most important factor for survival. Using a myeloablative preparative regimen and double UCBT, Brunstein et al66 showed comparable survival among patients receiving double UCBT, matched related donor, and matched or mismatched MUD HCT (Table 2). Ponce and colleagues67 reported similar survival among double UCBT, matched related donor, and MUD patients due to decreased late mortality in the double UCBT patients. Two retrospective RIC trials have shown comparable survival among MUD and double UCBT patients.68,69 In 2 parallel phase 2 trials of RIC double UCBT and haploidentical HCT (haplo), the 1-y DFS was comparable at 46% and 48%, respectively.70 TRM was higher after UCBT (24%) vs haplo (7%), but the relapse rate was lower after UCBT (31%) vs haplo (45%). A Clinical Trials Network randomized study is ongoing in the United States to compare long-term outcomes of the UCBT and haplo approaches.
Selected series comparing myeloablative single unit UCBT with transplantation of adult donors in adults
| Series . | Patients, n . | Conditioning . | Graft source . | Median Age (years) . | DFS, % . | Comment . |
|---|---|---|---|---|---|---|
| Takahashi, Blood 2007,95 hematologic malignancy | 71 | UCB | 38 | 70 | UCB similar or superior to matched sibling donor transplant | |
| 55 BM | Myelo | MRD | 40 | 60 | ||
| 6 PBSC | Ablative | |||||
| Atsuta, Blood 2009,96 acute leukemia | 287 | UCB | ||||
| 173 AML | Myelo | 38 | 42 | |||
| 114 ALL | Ablative | 34 | 46 | UCB similar to MUD in ALL | ||
| 533 | MUD | UCB inferior in AML (higher TRM) | ||||
| 311 AML | 38 | 54 | ||||
| 222 ALL | 32 | 44 | ||||
| Eapen, Lancet 2010,65 acute leukemia | 165 | UCB | 28 | 44 | UCB and MUD comparable; disease status only factor associated with DFS | |
| 888 | Myelo | PBSC | 39 | 50 | ||
| 623 | Ablative | MUD | ||||
| 265 | MMUD | |||||
| 472 | BM | 33 | 52 | |||
| 332 | MUD | |||||
| 140 | MMUD |
| Series . | Patients, n . | Conditioning . | Graft source . | Median Age (years) . | DFS, % . | Comment . |
|---|---|---|---|---|---|---|
| Takahashi, Blood 2007,95 hematologic malignancy | 71 | UCB | 38 | 70 | UCB similar or superior to matched sibling donor transplant | |
| 55 BM | Myelo | MRD | 40 | 60 | ||
| 6 PBSC | Ablative | |||||
| Atsuta, Blood 2009,96 acute leukemia | 287 | UCB | ||||
| 173 AML | Myelo | 38 | 42 | |||
| 114 ALL | Ablative | 34 | 46 | UCB similar to MUD in ALL | ||
| 533 | MUD | UCB inferior in AML (higher TRM) | ||||
| 311 AML | 38 | 54 | ||||
| 222 ALL | 32 | 44 | ||||
| Eapen, Lancet 2010,65 acute leukemia | 165 | UCB | 28 | 44 | UCB and MUD comparable; disease status only factor associated with DFS | |
| 888 | Myelo | PBSC | 39 | 50 | ||
| 623 | Ablative | MUD | ||||
| 265 | MMUD | |||||
| 472 | BM | 33 | 52 | |||
| 332 | MUD | |||||
| 140 | MMUD |
ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; PBSC, peripheral blood stem cell.
Comparison of survival after transplantation of double unit UCBT with that of adult donors
| Series . | Patients, n . | Conditioning . | Graft source . | Follow-up, y . | Median age, y . | Survival (PFS or DFS), % . | Comment . |
|---|---|---|---|---|---|---|---|
| Brunstein, Blood 2010,66 leukemia | 128 | Myelo | UCB | 5 | 25 | 51 | UCB similar to MRD, MUD |
| 204 | Ablative | MRD | 40 | 33 | |||
| 152 | MUD | 31 | 48 | ||||
| 52 | MMUD | 31 | 38 | ||||
| Ponce, BBMT 2011,67 heme malignancy | 75 | Myelo | UCBT | 2 | 37 | 55 | UCB similar to MUD |
| 108 | Ablative | MRD | 47 | 66 | |||
| 184 | MUD | 48 | 55 | ||||
| Brunstein, Blood 2011,69 leukemia and lymphoma | 50 | RIC | UCB | 1 | 58 | 46 | Now a randomized Clinical Trials Network study |
| 50 | haplo BM | 48 | 48 | ||||
| Chen, BBMT 2012,68 hematologic malignancy | 64 | RIC | dUCB | 3 | 53 | 30 | UCB similar to MUD |
| 221 | MUD | 58 | 40 |
| Series . | Patients, n . | Conditioning . | Graft source . | Follow-up, y . | Median age, y . | Survival (PFS or DFS), % . | Comment . |
|---|---|---|---|---|---|---|---|
| Brunstein, Blood 2010,66 leukemia | 128 | Myelo | UCB | 5 | 25 | 51 | UCB similar to MRD, MUD |
| 204 | Ablative | MRD | 40 | 33 | |||
| 152 | MUD | 31 | 48 | ||||
| 52 | MMUD | 31 | 38 | ||||
| Ponce, BBMT 2011,67 heme malignancy | 75 | Myelo | UCBT | 2 | 37 | 55 | UCB similar to MUD |
| 108 | Ablative | MRD | 47 | 66 | |||
| 184 | MUD | 48 | 55 | ||||
| Brunstein, Blood 2011,69 leukemia and lymphoma | 50 | RIC | UCB | 1 | 58 | 46 | Now a randomized Clinical Trials Network study |
| 50 | haplo BM | 48 | 48 | ||||
| Chen, BBMT 2012,68 hematologic malignancy | 64 | RIC | dUCB | 3 | 53 | 30 | UCB similar to MUD |
| 221 | MUD | 58 | 40 |
PFS, progression-free survival.
Future directions and the scientific basis of HSC function revisited
Better understanding of how to overcome the limitations of UCBT, such as delayed engraftment and poor immune cell reconstitution, is necessary to expand the indications of UCBT. A number of options are under laboratory and clinical assessment. One strategy would be to use perfusion of the placenta to collect more cells at the birth of a baby.71 Although potentially feasible, this is a time-consuming process that would not likely be of general use, except perhaps in selected UCB collection centers. Combining a haplo family or MUD with a single UCBT may provide adequate engraftment with either myeloablative or RIC conditioning, but this needs further investigation.72,73 Intra-marrow injection to bypass the homing process known to be highly inefficient after IV cell delivery has been attempted, with conflicting results.74,75 In a European study, intrabone injection had a significant advantage on engraftment with decreased GVHD.74 This was confirmed in a more recent publication comparing intrabone injection to double UCBT.76
Cord blood expansion and homing techniques
Multiple investigators have explored UCB expansion strategies as a way to augment the low cell dose. Delaney et al,77 using the notch ligand Delta-1, demonstrated expansion of short-term repopulating cells and an improvement in the time of neutrophil engraftment to 16 days. The MD Anderson group used a co-culture ex vivo with mesenchymal progenitor cells in 1 of 2 UCB units in 31 patients, reporting a 30-fold expansion in CD34+ count and median time to engraftment of 15 days.78 These results compare favorably with historical unmanipulated controls, who had a 24-day median engraftment. However, such clinical efforts require a double UCBT to ensure the presence of long-term engrafting HSC in the unmanipulated UCB. Some preliminary success has also been obtained using nicotinamide in combination with cytokines to ex-vivo expand CD 133+ UCB in the context of a double UCBT.79 Specialized centers would be required where the UCB can be expanded, and this adds substantially to the already additional costs of a double vs single UCBT. Other preclinical efforts to ex vivo expand HSCs have been reported.80,81
Other means to enhance the efficacy of UCBT are to increase the homing to and nurturing of cells within the hematopoietic microenvironment. Recent efforts include experimental studies using fucosylation of cells, inhibition of Dipeptidylpeptidase 4 (DPP4, expressed as CD26 on the cell surface), and pretreatment of donor cells with a modified Prostaglandin (PG) E molecule.82-86 One approach is the upregulation of CXCR4 expression, which is expressed on CD34+ progenitor cells, to increase marrow homing. One of 2 UCB units was incubated with PGE2; neutrophil engraftment improved by 3.5 days and the PGE2-treated UCB provided long-term hematopoeisis in 10 of 12 patients.85 The use of an FDA-approved, orally active inhibitor of DPP4 on engraftment of single unit UCBT in adults was initiated in adults with high-risk malignancies87 based on studies that demonstrated that DPP4 inhibition allows enhanced engraftment in animal studies,83,84 likely through effects on homing. However, inhibition of DPP4 can affect a number of cytokines produced by the BM-nurturing environment,88 as well as homing of HSC.83 Thus, modifications of the timing and duration of the oral DPP4 inhibitor in the clinical setting may be more effective than reported.87 Homing and nurturing procedures may be relatively inexpensive to perform and more widely used without extensive ex vivo maneuvers or experience. Combinations of these procedures may result in greater improvement in engraftment capacity than any one procedure itself. An example of a potential combination treatment is that of either PGE, cell fucosylation, or DPP4 inhibitor treatment of donor cells followed by the infusion of these cells into conditioned recipients that are given orally active DPP4 inhibitor prior to and following the UCB cell graft. As we learn more about the biology of HSCs and their responses to cytokines and cells of the hematopoietic microenvironment and as we determine how best to provide effective manipulation of these events, UCBT may become a more efficient and efficacious procedure.89
Conclusions
The field of UCBT has matured considerably over the last 25 years since the initial laboratory studies in Indiana and clinical work in Paris. Over 30 000 UCBTs have been performed. UCBT in children has similar or superior survival to a standard transplant, and results for adults continue to improve. Randomized studies to compare graft sources are underway. Much has been learned in a relatively short time about the properties of UCB HSCs and their clinical applications. All these results show that mismatched UCBT is feasible and might in the future achieve similar results to HLA-matched HCT. This is an evolving field that must be carefully evaluated with more comparative studies, which can be achieved by multicenter collaborations.90
UCBT needs to meet several new challenges. Delays in immune reconstitution have led to an increased incidence of late viral infections, which can be fatal, after UCBT.91,92 Many methods to improve the speed of engraftment and decrease TRM are under investigation, including an increased donor pool to decrease the number of HLA mismatches or the use of double UCBT. Other strategies in clinical trials are UCB intra-bone infusion, ex vivo expansion with cytokine cocktails, modification of homing and in vivo nurturing factors, and the use of mesenchymal stem/stromal cells. Lymphocyte subsets, Natural Killer cells, or mesenchymal stromal cells from UCB could be isolated and cultured and used for immunotherapy or cell repair. Induced pluripotent stem (iPS) cells can be generated from immature UCB cells,9,93,94 and it is possible that the future may allow expanded numbers of HSCs and HPCs from these iPS cells and generation of other cell types. There are concerns with the use of iPS or other cell types for future regenerative medicine attempts, but should these cells be obtained from UCB cells and be shown to be safe and effective as treatment modalities, it is likely that UCB will have enhanced usefulness. When we look back after the next 25 years, we anticipate an abundant UCB supply, digitalized UCB selection, multiple new indications, and significantly improved clinical outcomes.
Acknowledgments
The authors thank Drs Joseph Antin and Thomas Spitzer for their critical review of the manuscript.
Authorship
Contribution: K.K.B., E.G., and H.B. wrote the manuscript.
Conflict-of-interest disclosure: E.G. and H.B. are on the medical scientific advisory board of Corduse, a public and family cord blood banking company, and H.B. is a founder of the Corduse Family Cord Blood. H.B. has served as a consultant to Fate Therapeutics. The remaining author declares no competing financial interests.
Correspondence: Karen K. Ballen, Division of Hematology/Oncology, Massachusetts General Hospital Cancer Center, Zero Emerson, Suite 118, Boston, MA 02114; e-mail: kballen@partners.org.