Abstract
Treatment philosophies in multiple myeloma (MM) debate the relative merits of achieving the deepest possible remissions (“curative” doctrine) vs sequential delivery of antimyeloma agents (“control” doctrine). In this paper, we highlight the relevant strengths of each doctrine in the context of modern patient selection strategies, fresh biological insights on MM pathogenesis, agents with improved safety profiles, and emerging molecular and imaging tools. Paramount fundamental questions remain unanswered that require an intense research focus as we pursue a cure for this devastating disease.
Introduction: where are we today?
The treatment of multiple myeloma (MM) has witnessed remarkable progress since the implementation of proteasome inhibitors and immunomodulatory agents.1,2 Combining these agents with high-dose melphalan results in nonprogressive disease in a significant number of patients but is not universally tolerated.3 No integrated therapeutic strategy exists that reliably results in eradication of disease: a fact that earns MM the label of an incurable malignancy. The fundamental question regarding the curability of MM sparks much disagreement and controversy. Philosophical debates have scrutinized whether the goal of therapy should be an ardent pursuit of complete response (“curative” doctrine) or longitudinal management of a chronic disease that perpetually remits and relapses (“control” doctrine).4 At its core, the philosophical divide hinges on the efficacy and toxicity of the therapies used and requires careful reconsideration as agents evolve. Indeed, the inherent curability of MM remains the most profound unanswered fundamental question.
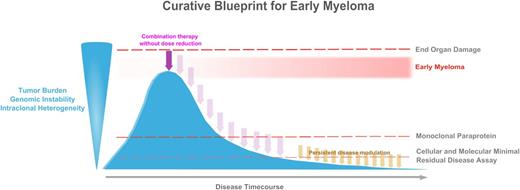
The pursuit of a breakthrough curative blueprint for MM is a justifiable concept, and the necessary components require definition. Elements of both treatment doctrines are critical for a curative blueprint because combinations of highly active agents are required to achieve maximal eradication of both founder and minor subclones (“curative” doctrine), and disease modulation after initial therapy will likely be required to extend response duration (“control” doctrine). These hypotheses must be rigorously studied in well-designed clinical trials prior to the widespread implementation of regimens without proven survival benefit. We propose a shift in research focus toward studying the effect of combination therapy delivered prior to overt organ dysfunction and advanced genomic complexity (ie, treatment of “early myeloma”) combined with highly sensitive methods of subclinical disease monitoring (Figure 1).
A curative blueprint for myeloma requires multiple components. The first step is to define patients with “early myeloma” and initiate therapy prior to end-organ damage. These patients would have less tumor burden, genomic instability, and intraclonal heterogenetity than patients with more advanced myeloma. Highly active combination therapy for early myeloma could be delivered without dose delay or dose reduction. Cellular and molecular assays for minimal residual disease (MRD) could be used to define disease eradication, guide strategies for persistent disease modulation (maintenance therapy), and monitor for relapse prior to appearance of a monoclonal paraprotein.
A curative blueprint for myeloma requires multiple components. The first step is to define patients with “early myeloma” and initiate therapy prior to end-organ damage. These patients would have less tumor burden, genomic instability, and intraclonal heterogenetity than patients with more advanced myeloma. Highly active combination therapy for early myeloma could be delivered without dose delay or dose reduction. Cellular and molecular assays for minimal residual disease (MRD) could be used to define disease eradication, guide strategies for persistent disease modulation (maintenance therapy), and monitor for relapse prior to appearance of a monoclonal paraprotein.
“Early myeloma”
A critical determinant of survival in most malignancies is early detection. Early detection is not applicable to MM, because there is no current definition of “early myeloma” and treatment protocols do not adapt therapy on the basis of tumor burden. MM is consistently preceded by a precursor state, rendering the effects of early intervention testable.5,6 Thus, one perspective holds that therapy for MM is delivered late in the course of genomic complexity in every case. Early intervention prior to clinical symptoms is not currently recommended, because persons with myeloma precursor diseases do not universally develop malignancy.7 The distinction between smoldering MM (SMM) and MM is not based on clear biological differences, however, and significant overlap exists. Determination of end-organ damage often requires subjective interpretation of insensitive and outdated modalities such as skeletal surveys. Hillengass et al8 recently reported that more than 30% of patients with SMM have bone marrow infiltration patterns similar to MM when using whole-body magnetic resonance imaging. An alternate proposal is to classify cases of MM with limited organ damage and SMM with the highest risk of progression as “early myeloma.” The concept of early myeloma requires reliable biological tools to distinguish cases of SMM that are “monoclonal gammopathy of undetermined significance–like” and those that are more “MM-like.” Treatment of early myeloma provides a unique opportunity to alter the natural history of MM but remains an unanswered fundamental question.
Biological impacts of early therapy
Genetic complexity
Genetic aberrations found in MM can be detected at a lower frequency within myeloma precursor states suggesting that progressive accumulation of acquired genetic events drives disease progression.9 One theory of pathogenesis contends that inciting genetic events are present in precursor states and remain plastic over time, whereas secondary genetic events result in a linear progression to MM.10 Recent data suggest a contrasting model of multiple subclones existing in dynamic equilibrium that alternate dominance over time akin to a Darwinian branching model of progression.11 Whole genome sequencing performed on a single patient during multiple clinical relapses confirmed molecular heterogeneity,12 but also demonstrated an evolutionary process that did not follow linear patterns. In a companion study, a branching, nonlinear pattern of alternating clonal dominance was observed across patients, whereas others demonstrated relatively stable genomes.13 Subclones demonstrated competition for survival with divergent sensitivity to individual agents that was altered with therapy. Whole exome sequencing of cases harboring the t(4;14) and t(11;14) translocations found differences in mutational patterns suggesting a unique evolutionary pathway for different subgroups.14 Taken together, these findings spawn an intriguing set of fundamental questions regarding the biological consequences of early interruption of this process of clonal evolution. Will treatment of early myeloma result in selection for resistant clones or subvert them entirely because fewer transformative genetic events will have occurred? Will treatment of early myeloma be most effective if combinations are used in an attempt to eradicate all subclones? Clinical trials with strong translational molecular investigations are needed to address these critical questions.
Plasma cell trafficking
A full understanding of early myeloma also requires characterization of the biological processes involved in plasma cell trafficking. Extensive extramedullary tumor burden is a poor prognostic sign, yet plasma cell trafficking outside the bone marrow compartment occurs frequently. Normal plasma cell maturation involves a continuous process of egress to other anatomical sites both within and outside of the bone marrow compartment. Ghobrial15 proposed a model of regional hypoxia within the bone marrow niche that unlocks homing to the bone marrow and promotes plasma cell egress and migration to distant sites. The burden of plasma cells within the bone marrow may contribute to regional hypoxia and has recently been identified as a risk factor for SMM progression.16 The relationship between malignant plasma cells and the cross talk with their microenvironment may distinguish early myeloma from more complex disease.
Barriers to treatment of early myeloma
Which patients?
A critical barrier to treating SMM is the absence of a biomarker that identifies which patients will unequivocally develop MM. Two groups have retrospectively identified variables that identify SMM patients at the highest risk of progression, but neither identifies individuals that will progress to MM with certainty.17,18 Additionally, these risk-stratification models for SMM have a low concordance rate with each other.19 Without precise tools for patient selection, the decision for early intervention centers on the perceived assessment of risk vs benefit. As therapies become more tolerable and effective, more patients and providers will be willing to accept the risks of toxicity (Figure 2). Age of the patient and presence of comorbidities will also influence this decision. Still, unintended long-term treatment-related risks may not be immediately apparent,20 and detailed long-term monitoring is mandatory. Highly robust biomarkers that precisely identify patients who will inevitably progress to MM are an essential component of shifting toward treatment of early myeloma.
The relationship of a regimen’s toxicity is dynamically related to its efficacy. As the regimens become more efficacious, more patients are willing to accept the risk of toxicity. Equally important, as the regimens become less toxic, the number of patients willing to undergo treatment increases. These treatment decision dynamics remain in constant flux as we move toward a curative regimen for early myeloma.
The relationship of a regimen’s toxicity is dynamically related to its efficacy. As the regimens become more efficacious, more patients are willing to accept the risk of toxicity. Equally important, as the regimens become less toxic, the number of patients willing to undergo treatment increases. These treatment decision dynamics remain in constant flux as we move toward a curative regimen for early myeloma.
Which treatment?
A major benefit of treating early myeloma is the delivery of maximal therapy with less dose interruption. By waiting until organs are compromised, we apply palliative doctrines to therapy that are more permissive of interruptions in treatment and reductions in dose intensity. The treatment of SMM that has been explored to date does not justify early intervention, because the toxicities of the therapies were too severe and the outcomes were not sufficiently compelling. Improved safety profiles of novel therapies now allow for combination approaches.
Mateos et al21 reported the effect of lenalidomide-dexamethasone followed by lenalidomide maintenance vs no treatment in patients with high-risk SMM. Of the first 51 patients to complete induction therapy, the overall response rate was 87% with 16% complete response (CR)/stringent CR. At a median follow-up of 32 months, the median time to progression in the observation arm was 23 months and was not yet reached in the experimental arm (P < .0001). A trend toward an overall survival benefit was reported with an estimate at 3 years of 98% compared with 82% (P = .05) in favor of treatment. These data serve as proof of principle that the treatment of high-risk SMM can be accomplished without excessive toxicity and may delay progression to MM.
Triplet combination regimens may better overcome the problem of intratumoral clonal heterogeneity.22 The immediately apparent downside, however, is the potential for irreversible toxicities. Remarkable results have recently been reported by Jakubowiak et al23 without severe toxicities. Using carfilzomib with lenalidomide and dexamethasone, 78% of patients who completed 8 cycles of therapy achieved near CR/CR; no patient reported G3/4 neuropathy. These results were recently confirmed by Korde et al,24 and all 10 patients who were assessed for presence of MRD utilizing multiparameter flow cytometry (MFC) were negative. It is enticing to consider the impact of initiating highly active combination therapy with full dose intensity prior to advanced genomic complexity and debilitating organ dysfunction. The treatment of SMM should still be restricted to clinical trials that emphasize translational end points until the fundamental questions that remain are addressed.
Response monitoring and disease surveillance
An innovative approach to characterizing response and disease surveillance is arguably the most important component of a curative blueprint for early myeloma. The current response criteria rely almost exclusively on the paraproteins in the blood and urine. Because contemporary combination therapy achieves near CR/CR in ∼75% of patients, these criteria must also advance. In this way, important concepts from the control doctrine become applicable because most patients are expected to relapse, and long-term disease modulation may be required to extend that remission. MRD assessment may identify patients who benefit the most from a given therapy and identify those at highest risk for progression. At this point, however, no data exist to support treatment of early molecular relapse. Improved standardized methods of measuring molecular responses to therapy provide an opportunity to further risk-stratify patients after initial therapy.
The achievement of MFC remission appears more prognostic than conventional definitions of CR. Paiva et al25 demonstrated that in patients who achieved CR after high-dose therapy, MRD detected by MFC predicted a higher risk for relapse than those who became MRD negative. In patients treated without high-dose therapy, the same group demonstrated that MFC similarly provided more prognostic information than achievement of CR.26 Molecular definitions of remission have also been explored using polymerase chain reaction (PCR) assays to detect rearrangements in the immunoglobulin heavy chain (IgH) gene. Allele-specific oligonucleotide PCR, fluorescent PCR, and allele-specific oligonucleotide real-time quantitative PCR techniques are more sensitive than MFC, but they are expensive, labor intensive, and only applicable in 60% to 75% of MM patients due to somatic hypermutation in the IgH variable region.27 The standardization of MRD techniques and universal reporting in clinical trials is a critical and necessary advancement.
Importantly, MRD status must be evaluated in the context of novel response imaging because they may be incongruent. MM is a patchy disease of the bone marrow, yet current assessment of disease burden focuses on imaging of the bony cortex. Focal lesions (FL) as detected by whole-body magnetic resonance imaging are often present before therapy and may persist for months posttherapy.28 The number and distribution of FLs after therapy is prognostic, and the resolution of FLs is associated with superior overall survival, whereas persistence of FLs identifies patients at high risk of relapse.28,29 Similarly, positron emission tomography scanning has been used to identify patients with a high risk of relapse after therapy,30 but current radiotracers are not MM specific and do not have a clear role in disease monitoring. Improvements in anatomical and functional imaging have the potential to play a crucial role in the diagnosis of early myeloma, response assessment, and disease monitoring.
Conclusion and future directions
The inherent curability of MM is a fundamental unanswered question and the source of much controversy. Current philosophical perspectives debate curative vs control doctrines that are not dichotomous. In this paper, we highlight the necessary components of each doctrine that could form the backbone of an integrated curative blueprint for early myeloma. An aggressive pursuit of the curative blueprint requires shifting the research focus toward the biological basis for patient selection, combination therapy prior to advanced genomic complexity (molecular profiling), and monitoring response with advanced methods aimed at defining disease eradication (assays for MRD). We believe that the time has come to directly address these challenges and test the paradigm of treating early myeloma in a scientifically rigorous and collaborative fashion.
Acknowledgment
This work was supported by the intramural research program of the National Institutes of Health.
Authorship
Contribution: M.R., N.K., S.P.W., and O.L. designed the figures and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Ola Landgren, Multiple Myeloma Section, Center for Cancer Research, Metabolism Branch, National Cancer Institute, National Institutes of Health, 9000 Rockville Pike, Building 10/Room 13N240, Bethesda, MD 20892; e-mail: landgreo@mail.nih.gov.