In this issue of Blood, Ross et al report the TWISTER study in which imatinib was stopped in chronic myeloid leukemia patients with undetectable minimal residual disease (UMRD) and show that >40% remained off treatment without recurrence at 2 years.1
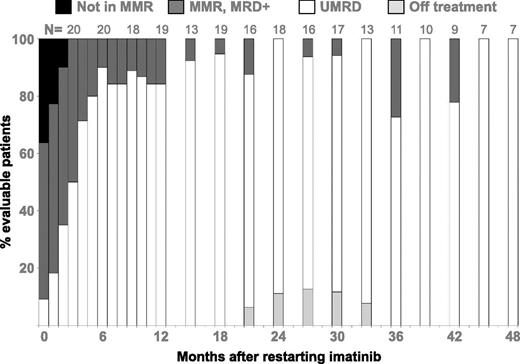
Response to retreatment with IM after molecular relapse. Four years of follow-up after molecular relapse is shown. The number of evaluable patients at each time point is shown at the top of the column. Because of the smaller number of observations at later time points, the data after 12 months are grouped to show the 3 months prior to the stated time point (ie, results for 15 months are all results for the interval 13-15 months). If a patient had two qRT-PCR results in an interval, the patient is scored only once with the highest recorded BCR-ABL level. MMR, major molecular response; MRD, detectable minimal residual disease. This is Figure 2 from the article by Ross et al that begins on page 515.
Response to retreatment with IM after molecular relapse. Four years of follow-up after molecular relapse is shown. The number of evaluable patients at each time point is shown at the top of the column. Because of the smaller number of observations at later time points, the data after 12 months are grouped to show the 3 months prior to the stated time point (ie, results for 15 months are all results for the interval 13-15 months). If a patient had two qRT-PCR results in an interval, the patient is scored only once with the highest recorded BCR-ABL level. MMR, major molecular response; MRD, detectable minimal residual disease. This is Figure 2 from the article by Ross et al that begins on page 515.
This is the first prospective study to confirm the observations of the Stop Imatinib (STIM) study, in which 40% of patients who had achieved deep molecular responses (MRs) on imatinib could discontinue the drug without experiencing relapse,2 which led to speculation that “cure” could be achieved by using oral tyrosine kinase inhibitors (TKIs) alone. Subsequently, randomized phase 3 studies comparing the more potent second generation TKI (2GTKI) with imatinib as first-line therapy showed that these very deep responses were obtained more rapidly and probably in a higher proportion of patients than with imatinib3,4 and resulted in the dilemma currently besetting chronic myeloid leukemia patients and their physicians, namely the choice of the first-line agent.
In STIM, patients were carefully selected and had undetectable disease for a minimum of 2 years, as measured by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) assays at their local laboratory. Furthermore, this negative result was confirmed before study entry by the central laboratory where the qRT-PCR assay was capable of detecting 5-log reductions in BCR-ABL1 transcript levels. Many patients had been negative by qRT-PCR for considerable periods of time (median, 36 months; range, 24 to 85 months). Since then, successful discontinuation of TKIs has been reported retrospectively, but now, Ross et al have reproduced the STIM results in a prospective study of 40 patients with UMRD by using a qRT-PCR assay with a sensitivity of 4.5 log.
Eligibility criteria were similar to those in STIM, with a minimum of 3 years of imatinib and a minimum of 2 years of UMRD. The primary end point of treatment-free remission (TFR) at 2 years was met by 47% of the patients. Fifteen of the 22 patients who relapsed did so within 6 months, and all relapsing patients responded promptly with similarly deep MRs on reintroduction of imatinib (see figure), a reassuring fact for any patient contemplating treatment cessation. qRT-PCR for bone marrow offered no advantage over qRT-PCR for peripheral blood with respect to predicting or identifying early relapse, another welcome finding. This group previously investigated the role of patient-specific BCR-ABL1 DNA PCR5 in identifying residual disease in patients with transcripts undetectable by qRT-PCR. BCR-ABL1 DNA was identified in all the evaluable patients in TFR, suggesting that this test is not predictive for the ability to remain off treatment and that mechanisms other than direct inhibition of Bcr-Abl1 are responsible for continuing remission.
Patients who had previously received interferon were more likely to remain in TFR. Whether this simply reflects the fact that patients who received interferon and survived to receive imatinib had an inherently good prognosis (an explanation supported by qRT-PCR results compatible with at least partial cytogenetic responses at study entry in 16 of 21 patients) or suggests a beneficial effect of interferon exposure or both is unclear. Interestingly, for patients treated with imatinib alone, only 33% remained in TFR at 2 years. For this group, time to UMRD was not predictive of TFR, a fact that argues against the use of 2GTKI upfront to obtain early deep responses.
Readers may be familiar with a popular children’s game called Bop It!, in which players are encouraged to “twist it” and “spin it.” We should be careful to avoid the “spin” that might ensue from this report. As the authors acknowledge, the completeness of MR is dependent on the assay sensitivity. Few laboratories worldwide have robust qRT-PCR, and even fewer have access to international standardization allowing them to report results on the international scale.6 TWISTER and STIM show that 30% to 40% of patients achieving durable MR4.5 or MR5 on imatinib can cease treatment for a prolonged period of time, but we must be wary of extrapolating these findings to patients with less deep responses, especially if the laboratory is unable to report the response on the international scale. Discontinuation in these patients is rapidly followed by relapse and difficulties in establishing deep responses on reintroducing treatment.7 Patients suitable for stopping treatment should have assessments at high-quality laboratories and have monthly qRT-PCR for at least 1 year in order to identify and treat early relapse.
The high price of the second- and third-generation TKI is often justified by the suggestion that by treating patients with the more potent drugs, more patients will be able to stop therapy. However, relatively few patients achieve deep and sustainable responses that would render them eligible for a stopping study similar to STIM or TWISTER. Milojkovic et al8 reported that the proportion of patients achieving sustained MR for a period of 2 years would depend on whether the requirement was MR4, MR4.5, or MR5. In 360 patients treated with imatinib in chronic phase, approximately 30% achieved sustained MR4 but only 19% attained MR5. Deep responses were more likely in females and in those who had not required treatment with 2GTKI. The Adelaide group9 studied 423 chronic phase patients treated with imatinib from diagnosis and found a cumulative incidence of MR4.5 sustained for 2 years of 36.5% at 8 years. Factors predicting for this included female sex and the qRT-PCR level 3 months after treatment initiation. These data together with those from TWISTER suggest that 12% of patients treated with imatinib will eventually be able to discontinue therapy. In absolute figures, however, 121 of the 423 patients achieved MR4.5. Eighty had 24-month follow-up and, of these, only 51 (12% of the original 423) sustained this response for 2 years—and many must have entered TWISTER. Admittedly, few patients achieve MR4.5 in the first years of treatment, so the proportion reaching MR4.5 will increase over time. But we are left with the fact that after 14 years of imatinib therapy, less than 5% of patients have been able to durably discontinue therapy. Even if 2GTKI used early in treatment doubled this figure, it seems that life-long therapy will be required for the majority of patients and we, as their physicians, must be realistic in our expectations.
Conflict of interest disclosure: J.F.A. has received honoraria for speaking in satellite symposia and for attending advisory boards for Ariad Pharmaceuticals, Bristol-Myers Squibb, Novartis, and Pfizer.