To the editor:
We have read with great interest the article from Döbel et al,1 which shows the ability of freshly separated 6-sulfo LacNac (slan)–positive dendritic cells (slanDCs) to internalize immunoglobulin G (IgG)-opsonized particles and aggregated IgG via FcγRIIIA/CD16-dependent phagocytosis. SlanDCs are a subset of “nonclassical monocytes” expressing CD16.2,3 Schäkel et al4 have previously elegantly demonstrated that these cells in vitro undergo rapid spontaneous maturation, becoming potent producers of interleukin-12, and that these events in vivo are restrained by CD47 expressed on the membranes of erythrocytes; now, the same group reveals that metalloprotease-dependent shedding of the low-affinity IgG receptor CD16 accompanies these changes.1
We noted the downregulation of CD16 in all CD16-expressing mononuclear myeloid cells in experiments analyzing the cytokine response to Toll-like receptor (TLR) 4 and TLR7/8 agonists in lithium-heparinized whole blood (and thus in the presence of erythrocytes) (Figure 1A-C). As shown in Figure 1C, this modulation is concomitant with the production of tumor necrosis factor α (TNFα). Thus, microbial stimuli can bypass the restraining effect mediated by erythrocytes on the maturation of nonclassical monocytes.
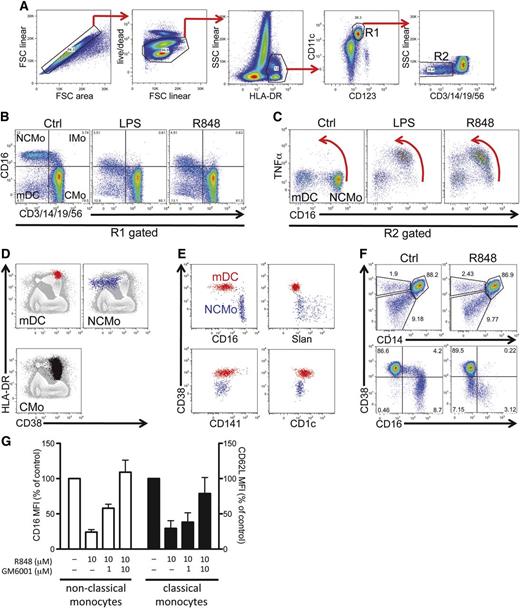
Alternative identification of nonclassical monocytes for the study of the mechanism underling CD16 shedding. (A) Gating strategy used in a representative intracellular cytokine staining experiment on whole-blood stimulations. Cells are selected via sequential gating by the exclusion of doublets and dead cells followed by the positive gating on HLA-DR and CD11c (myeloid mononuclear cells, gate R1). A further gate on lineage-negative cells (R2) is set to select both mDCs and nonclassical monocytes. (B) R1 gated cells are plotted for control, LPS (0.5 μg/mL), and R848 (10 μM) (both reagents from Invivogen) stimulated tubes in a lineage vs CD16 plot to visualize the decrease in CD16 fluorescence intensity following activation in a visualization mode commonly used to identify monocyte subtypes: CMo, IMo, and NCMo. (C) Downregulation of CD16 in NCMo is concomitant with the production of TNFα. Red arrows emphasize the concomitant intracellular accumulation of TNFα and loss of surface CD16. (D-E) Distribution of CD38 in different myeloid mononuclear populations. mDC (red), NCMo (blue), and CMo (black) identified essentially as described in panels A through C are overlaid on total mononuclear cells (gray) in HLA-DR vs CD38 plots (D). (E) Segregation of DC subsets defined by the expression of CD16, slan, CD141, and CD1c into CD38-positive and -negative lineageneg myeloid mononuclear cells. (F) In the top 2 panels, HLA-DR+CD11c+ cells were plotted in CD14 vs CD38 bivariate plots for both Ctrl- and R848- (10μM, 1 hour) stimulated tubes to show how mDC, NCMo, and CMo can be segregated one from another by the use of CD38. The bottom 2 plots illustrate the decrease in CD16 expression occurring following stimulation with R848; CD38, in contrast, remains unchanged. (G) Dose-dependent inhibitory effect of the panmetalloprotease inhibitor GM6001/Ilomastat (Sigma-Aldrich) on CD16 (□) and CD62L (▪) shedding induced by 1-hour incubation with R848 (both reagents were added at the same time at the starting of the culture; bars are means, and error bars represent SD of 3 experiments from 3 distinct donors). Cells are gated on CD11c+HLADR+; CMo are identified as being CD14+CD38+, while nonclassical monocytes are CD14low/−CD38−. Cytometric off-line analysis was performed with FlowJo software (Tree Star), and generated numerical data graphed in Prism (GraphPad). Ctrl, control; CMo, classical monocyte; FSC, forward scatter; IMo, intermediate monocyte; LPS, lipopolysaccharide; mDC, myeloid dendritic cells; MFI, mean fluorescence intensity; NCMo, nonclassical monocyte; SSC, side scatter.
Alternative identification of nonclassical monocytes for the study of the mechanism underling CD16 shedding. (A) Gating strategy used in a representative intracellular cytokine staining experiment on whole-blood stimulations. Cells are selected via sequential gating by the exclusion of doublets and dead cells followed by the positive gating on HLA-DR and CD11c (myeloid mononuclear cells, gate R1). A further gate on lineage-negative cells (R2) is set to select both mDCs and nonclassical monocytes. (B) R1 gated cells are plotted for control, LPS (0.5 μg/mL), and R848 (10 μM) (both reagents from Invivogen) stimulated tubes in a lineage vs CD16 plot to visualize the decrease in CD16 fluorescence intensity following activation in a visualization mode commonly used to identify monocyte subtypes: CMo, IMo, and NCMo. (C) Downregulation of CD16 in NCMo is concomitant with the production of TNFα. Red arrows emphasize the concomitant intracellular accumulation of TNFα and loss of surface CD16. (D-E) Distribution of CD38 in different myeloid mononuclear populations. mDC (red), NCMo (blue), and CMo (black) identified essentially as described in panels A through C are overlaid on total mononuclear cells (gray) in HLA-DR vs CD38 plots (D). (E) Segregation of DC subsets defined by the expression of CD16, slan, CD141, and CD1c into CD38-positive and -negative lineageneg myeloid mononuclear cells. (F) In the top 2 panels, HLA-DR+CD11c+ cells were plotted in CD14 vs CD38 bivariate plots for both Ctrl- and R848- (10μM, 1 hour) stimulated tubes to show how mDC, NCMo, and CMo can be segregated one from another by the use of CD38. The bottom 2 plots illustrate the decrease in CD16 expression occurring following stimulation with R848; CD38, in contrast, remains unchanged. (G) Dose-dependent inhibitory effect of the panmetalloprotease inhibitor GM6001/Ilomastat (Sigma-Aldrich) on CD16 (□) and CD62L (▪) shedding induced by 1-hour incubation with R848 (both reagents were added at the same time at the starting of the culture; bars are means, and error bars represent SD of 3 experiments from 3 distinct donors). Cells are gated on CD11c+HLADR+; CMo are identified as being CD14+CD38+, while nonclassical monocytes are CD14low/−CD38−. Cytometric off-line analysis was performed with FlowJo software (Tree Star), and generated numerical data graphed in Prism (GraphPad). Ctrl, control; CMo, classical monocyte; FSC, forward scatter; IMo, intermediate monocyte; LPS, lipopolysaccharide; mDC, myeloid dendritic cells; MFI, mean fluorescence intensity; NCMo, nonclassical monocyte; SSC, side scatter.
Extending the observations of Belge et al5 who proposed HLA-DR as a stable marker for nonclassical monocytes, to track the loss of CD16 in monocyte subsets we developed a flow cytometric strategy which identifies nonclassical monocytes, together with other monocyte and myeloid DC subsets, taking advantage of the differential expression pattern of CD38, thus bypassing the need for CD16 as a critical selection marker. Nonclassical monocytes express CD38 at very low levels, unlike other monocytes and DC subsets (Figure 1D-E), consistent with their proposed developmental derivation from classical monocytes3 and their well-differentiated phenotype.
Because CD38 expression is stable, at least following short-term stimulation (Figure 1F), we have exploited this strategy to monitor CD16 downregulation in nonclassical monocytes and, as a control, CD62L shedding in classical monocytes6 after stimulation with TLRs. As shown in Figure 1G, the metalloprotease inhibitor Ilomastat (GM6001) dose-dependently prevents CD16 and CD62L shedding induced by the brief incubation with resiquimod (R848, a TLR7/8 agonist).
In conjunction with the results presented by Döbel et al,1 our data thus demonstrate that the process of ectodomain shedding of 2 distinct surface receptors occurs in the same circumstances and by overlapping mechanisms in both nonclassical monocytes (and slanDCs) and classical monocytes, underscoring the similarities between the activation-dependent enzymatic machinery of 2 closely related monocyte family members. Furthermore, we show that TLR engagement can drive a response that overcomes the inhibition exerted by red blood cells in nonclassical monocytes. Our data extend the findings from Döbel et al not only to nonclassical monocytes other than slanDCs, but also to the whole monocyte family.
Nonclassical monocytes and circulating slanDCs are expanded during sepsis and HIV viremia.7-9 These “patrolling monocytes”10 are physiologically present in the pool of blood leukocytes crawling along the luminal side of vessels, and their spontaneous activation is restrained by erythrocytes. Our evidence suggests that the systemic spreading of microbial products occurring in pathological conditions can bypass this constraint, and that the novel marker CD38 can be successfully used to track recently activated nonclassical monocytes that, irrespective of expression of slan, have lost their defining marker CD16.
Authorship
Acknowledgment: This work was supported by Italian Ministry of Health and by the Italian Multiple Sclerosis Foundation (L.B.).
Contribution: M.P. designed and performed experiments, analyzed the data, and wrote the manuscript; L.B. provided funding, contributed to data interpretation and edited the manuscript; and G.B. supervised the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giovanna Borsellino, Santa Lucia Foundation, Istituto Di Ricovero e Cura a Carattere Scientifico, Via del Fosso di Fiorano 65, Rome, 00143 Italy; e-mail: g.borsellino@hsantalucia.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal