In this issue of Blood, McGrath et al show that the terminal glycan structures of platelet von Willebrand factor (VWF) are markedly different compared with such structures present on plasma VWF.1 Unexpectedly, these differences endow platelet VWF with a specific resistance against proteolysis by the VWF-cleaving protease ADAMTS13, thereby potentially increasing the hemostatic potential of platelet VWF during the formation of platelet-rich thrombi.
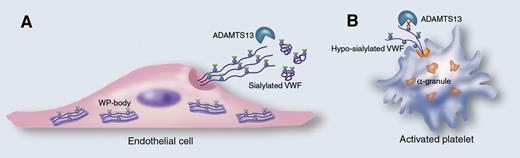
Cellular source of VWF determines its susceptibility for ADAMTS13-mediated proteolysis. VWF produced by endothelial cells (A) is stored in the Weibel-Palade (WP) bodies as a glycoprotein containing N-linked glycans (blue dots) that are sialylated (green triangles). Upon stimulated release, VWF assembles into long strings that are susceptible to proteolysis by ADAMTS13. Unexpectedly, McGrath et al have now found that VWF stored in the α-granules of platelets (B) contains much less sialic acids on the N-linked glycans (blue dots). Moreover, this lack of sialic acids converts VWF into a substrate that is less efficiently proteolyzed by ADAMTS13, providing it with a higher hemostatic potential. Professional illustration by Marie Dauenheimer.
Cellular source of VWF determines its susceptibility for ADAMTS13-mediated proteolysis. VWF produced by endothelial cells (A) is stored in the Weibel-Palade (WP) bodies as a glycoprotein containing N-linked glycans (blue dots) that are sialylated (green triangles). Upon stimulated release, VWF assembles into long strings that are susceptible to proteolysis by ADAMTS13. Unexpectedly, McGrath et al have now found that VWF stored in the α-granules of platelets (B) contains much less sialic acids on the N-linked glycans (blue dots). Moreover, this lack of sialic acids converts VWF into a substrate that is less efficiently proteolyzed by ADAMTS13, providing it with a higher hemostatic potential. Professional illustration by Marie Dauenheimer.
Glycosylation is a posttranslational modification that involves more than 50% of the members of the eukaryotic proteome and results in the covalent attachment of carbohydrate structures to the protein backbone. This type of posttranslational modification is a crucial event because it may affect different steps within the lifecycle of a protein—from biosynthesis to function and ultimately to clearance. Impaired glycosylation can be associated with severe clinical manifestations, including neonatal death, psychomotor retardation, cardiomyopathy, and coagulopathy.2 Whereas glycosylation is relying on an intact machinery to attach the correct glycan structures to the protein, environmental factors such as age, diet, and pathogen infections can also influence this process. In addition, another important determinant of glycosylation is the cellular origin of a protein because the available repertoire of enzymes mediating glycosylation varies per cell type. This may, for instance, affect the antennary (or branches) of the glycans, but also the terminal structures of the carbohydrates, such as the extent of sialylation or the absence or presence of blood group determinants.
McGrath et al present such an example in which the glycosylation pattern of a protein (in this case, VWF) is dependent on its cellular origin.1 VWF is a multimeric protein that is pivotal to the recruitment of platelets to the injured vessel wall and subsequent thrombus growth. Its expression is limited to 2 cell types. First, the major part of VWF is produced in endothelial cells (representing approximately 85% of all VWF), where it is either constitutively secreted in plasma or stored in the Weibel-Palade bodies. The remaining VWF protein is synthetized and stored in the α-granules of megakaryocytes and platelets, with little (if any) constitutive secretion from these cells. Consequently, circulating plasma VWF primarily originates from endothelial cells, whereas VWF in α-granules is selectively released upon platelet activation. Based on its presence in the circulation, plasma VWF is thought to be more dominant in the hemostatic process than platelet VWF. Indeed, the contribution of platelet VWF to hemostasis has remained controversial. For instance, platelet VWF is absent in dogs, without any obvious hemorrhagic consequences. However, patient observations and a recent crossover study using wild-type and Vwf-deficient mice revealed that platelet VWF can contribute to the hemostatic process as well.3,4
Despite having a similar protein backbone, plasma and platelet VWF have been reported to display subtle functional differences.5 Whereas both sources of VWF bind similarly to collagen, platelet VWF appears more efficient in binding to integrin αIIbβ3 and to heparin and, in contrast, less efficient in binding to the platelet-receptor glycoprotein Ibα.6 Because the protein backbone is similar between plasma and platelet VWF, it seems conceivable that variations in the glycosylation pattern may contribute to such functional differences. Indeed, initial analysis revealed that platelet VWF contains less sialic acids and lacks the blood group A and B glycan structures that are found on plasma VWF.5,6
In the present study, McGrath et al confirm the observation that sialylation of N-linked glycans (but not O-linked glycans) is reduced by more than 50% for platelet VWF compared with plasma VWF (see figure). As expected, blood group A- and B-glycan structures were absent but surprisingly enough, blood group O structures were normally present. Why specifically the A- and B-glycan structures are missing remains unclear, because other platelet proteins do contain these structures, suggesting that the necessary machinery is available during VWF protein synthesis in megakaryocytes.
Previously, it has been reported that ABO blood group structures modulate the efficiency by which VWF is degraded by its cleaving protease ADAMTS13, with O-structures rendering VWF more susceptible to cleavage than A or B structures.7 Because platelet VWF selectively carries the blood group O-structures, one would expect that it is degraded efficiently by ADAMTS13. However, McGrath et al observed the opposite: platelet VWF is much more resistant against ADAMTS13-mediated proteolysis than plasma VWF. Apparently, the explanation behind this paradoxical finding lies in the reduced quantity of sialic acids that are attached to the N-linked glycans of platelet VWF. Indeed, previously, the same group reported that α2,6-linked sialic acids promote proteolysis by ADAMTS13; a reduction of >50% of these sialic acids could explain why platelet VWF is proteolyzed less efficiently by ADAMTS13. It further indicates that the role of sialic acids is more dominant than the role of the blood group determinants in regulating ADAMTS13-mediated proteolysis. This would be in agreement with sialic acids being much more abundant (>90% of the glycans being sialylated in plasma VWF) than the blood group ABO structures (1-2 per monomer).8
Altogether, this article reveals new insights into platelet VWF with the observation that its glycome is markedly different from plasma VWF, providing it with a higher resistance to ADAMTS13 proteolysis. However, this report also highlights how much we have yet to learn about this particular VWF that, despite being highly multimerized and ADAMTS13-resistant, is less efficient than plasma VWF in binding to its platelet receptor glycoprotein Ibα. Additional studies focusing on platelet VWF–specific functions are necessary to further unveil its mysteries.
Conflict-of-interest: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal