Key Points
Commitment to the erythroid transcriptional program precludes endothelial development in a blood island precursor population.
Abstract
The developmental relationship between the blood and endothelial cell (EC) lineages remains unclear. In the extra-embryonic blood islands of birds and mammals, ECs and blood cells are closely intermixed, and blood island precursor cells in the primitive streak express many of the same molecular markers, leading to the suggestion that both lineages arise from a common precursor, called the hemangioblast. Cells within the blood island of Xenopus also coexpress predifferentiation markers of the blood and EC lineages. However, using multiple assays, we find that precursor cells in the Xenopus blood island do not normally differentiate into ECs, suggesting that classic hemangioblasts are rare or nonexistent in Xenopus. What prevents these precursor cells from developing into mature ECs? We have found that bone morphogenetic protein (BMP) signaling is essential for erythroid differentiation, and in the absence of BMP signaling, precursor cells adopt an EC fate. Furthermore, inhibition of the erythroid transcription pathway leads to endothelial differentiation. Our results indicate that bipotential endothelial/erythroid precursor cells do indeed exist in the Xenopus blood island, but BMP signaling normally acts to constrain EC fate. More generally, these results provide evidence that commitment to the erythroid lineage limits development of bipotential precursors toward an endothelial fate.
Introduction
The large majority of endothelial cells (ECs) within the embryo arise as individual angioblasts throughout mesodermal tissues. If hemangioblasts, bipotential precursors of blood and ECs (B/ECs), do indeed exist, they are located in those regions where blood and endothelial precursors develop in close proximity; for example, in the primitive streak and early blood island of the avian1 and mammalian embryos, and presumably within the primitive blood-forming regions of fish and amphibian embryos. Because blood and endothelium are both mesoderm derivatives, it is important to define the putative hemangioblast as an immediate precursor of these 2 lineages and not simply as an early mesodermal cell.
The hemangioblast theory was bolstered by molecular evidence that blood and endothelial precursors cells express an overlapping set of marker genes. For example, the cell surface receptor, Kdr, and the transcription factors, Erg, Etv2, Fli1, Gata1/2, Tal1, and Lmo2, are expressed in both blood and endothelial precursors.2-6 In vitro studies have demonstrated that cells isolated from mouse embryoid bodies and the primitive streak can generate blood and ECs in clonal culture,2,7-9 and similar results have been obtained using human embryonic stem cells.3 Although these studies show that cultured single cells can give rise to multiple cell lineages, including blood and endothelium, it has been much more challenging to demonstrate the existence of such cells within the embryo itself.
Several studies searching for embryonic hemangioblasts reached different conclusions about the existence, or at least the frequency, of such cells in vivo.10-13 Photoactivation of lineage tracers in the zebrafish embryo showed that single cells within the ventral marginal region of the gastrula could generate both blood and endothelial derivatives. It was estimated that as many as 12% of cells in this region had properties consistent with the hemangioblast.10 In contrast, lineage tracing of cells in the mouse blood islands failed to reveal any cells that generated both blood and endothelial progeny from a single clone.11 Although it is not possible to demonstrate the nonexistence of a cell type, the authors concluded that if hemangioblasts exist, they must be exceedingly rare.11 Numerous subsequent studies have failed to generate consensus on the existence of hemangioblasts in vivo, primarily due to questions concerning experimental design or interpretation of results.
In this study, we tested for the presence of hemangioblasts in the ventral blood island (VBI) of Xenopus, which provides a uniquely accessible and localized precursor population. Although markers of both endothelial and blood precursor cells are coexpressed in the VBI, our data demonstrate that these cells become blood and do not normally give rise to ECs, effectively precluding the existence of a classic bipotential precursor. However, experimental inhibition of bone morphogenetic protein (BMP) signaling blocks erythroid development and reveals a latent capacity for early VBI cells to differentiate into ECs. Furthermore, blocking the erythroid transcriptional program resulted in endothelial differentiation. These results indicate that precursor cells in the Xenopus VBI possess both endothelial and erythroid potential, but that in normal development, these cells are not hemangioblasts as such and give rise only to blood. These results may help to explain contradictory results in the literature and aid in understanding the endothelial/blood lineage decision in higher vertebrates.
Materials and methods
Embryo manipulation and inhibitor treatment
Embryos were cultured in 0.2× Marc's Modified Ringer (MMR), and at stage (st)12 or 17, embryos were moved to 0.6× MMR for surgical manipulation and healing for 6 to 8 hours, after which they were moved back to 0.2× MMR for subsequent development. Hand-made tungsten wire tools were used to excise the VBI or ventral posterior (VP) portion of the embryo. Inhibitors were dissolved in dimethylsulfoxide, and dose curves were performed to find the optimal potency without developmental defects. Embryos were treated with BMP inhibitors LDN-193189 (Cellagen) at 50 μM and DMH1 (Calbiochem) at 20 μM, NOTCH inhibitor RO4929097 (Cellagen) at 50 μM, WNT inhibitor XAV939 (Tocris) at 100 μM, fibroblast growth factor inhibitor SU50492 (Calbiochem) at 10 μM plus 0.1 mM ATP,14 and vascular endothelial growth factor (VEGF) inhibitor Ki8751 (Calbiochem) at 10 μM beginning at st13. This study received Institutional Animal Care and Use Committee approval from the University of Arizona (approval number 08-044).
Preparation of mRNA and in situ probes
Coding regions of Xenopus laevis gata2 (BC108544, ID# 7978680), lmo2 (BC097502, ID# 4174203), and tal1 (BC072130, ID# 4175038) were inserted into pT7TS expression plasmid for mRNA synthesis. Plasmids were linearized with SalI or XbaI, and mRNA was synthesized using T7 RNA polymerase (Ambion). Histone H2B–red fluorescent protein (RFP) plasmid, VEGF,15 and Noggin (EXRC clone KL-150) were linearized with NotI, and mRNA was synthesized with SP6 RNA polymerase (Ambion). Antisense digoxigenin-labeled probes were prepared and visualized using standard protocols with further detail available on request.
Morpholino and mRNA injections
The tal1 antisense morpholino (MO) (60 ng) (tal1 MO, 5′ ACAGGGACTTCTACTACCTCTCCAA 3′) and lmo2 antisense MO (60 ng) (lmo2 MO, 5′ TACAGTAGTCGATATCTCTCTTTCT 3′) are complementary to the X. laevis translational start site of each gene. The gata2 antisense MOs (25 ng total, 12.5 ng each MO; gata2a MO 5′ CTTCCATCGCAGGAGCAAAGTTCTC 3′, gata2b MO 5′ GGTCAGTAGCCACTTCCATTGCAGG 3′) and etv2 MO have been reported previously.5,16,17 The etv2, tal1, and lmo2 MOs were all injected at the 1-cell stage. The gata2 MOs were targeted ventral injections at the 4-cell stage. Noggin mRNA injections (total, 25 pg) were targeted to ventral regions of the embryo.
Immunofluorescence and imaging
Donor embryos were injected at the 1-cell stage with 500 pg of mRNA encoding H2B-RFP, and labeled VBIs were transplanted to wild-type hosts at st17. Fluorescent imaging was performed on a Leica M205 FA. Migratory cells were manually tracked using the ImageJ plug-in MTrackJ.18 Embryos of the Flk1::eGFP transgenic line19 were injected with 500 pg of mRNA encoding H2B-RFP, and VBIs were transplanted to wild-type recipients at st17. Mouse anti-green fluorescent protein (GFP; Invitrogen) and rabbit anti-RFP (Invitrogen) were used at 1:250 following standard procedures.20
Results
Removal of the blood island results in loss of hematopoietic marker expression but endothelial patterning is unaltered
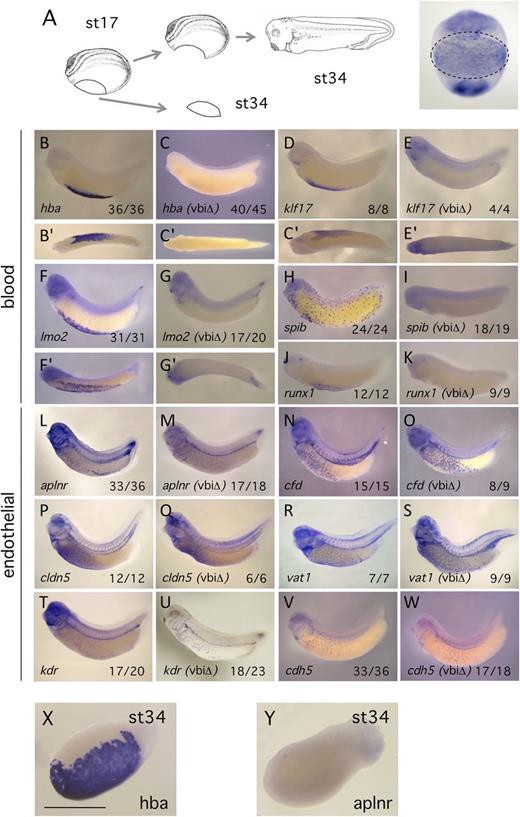
Similar to the blood island precursor populations of other organisms,2,3 markers of both the endothelial and blood lineages are coexpressed in the early VBI of Xenopus4,5,17,21 (supplemental Figure 1 available on the Blood Web site). To our knowledge, however, no study has investigated whether ECs ultimately differentiate within, or arise from, this region. To explore the contribution of the VBI precursor population to the developing B/EC lineages, we surgically excised the entire blood island tissue at stage 17 (Figure 1A), when markers of both lineages are highly expressed4 and prior to any migration of cells from the region22 (and see below). Excision of the VBI resulted in complete elimination of hematopoietic marker expression from the tailbud stage embryo (Figure 1B-K). These results show that the excisions were accurate, and more importantly, the absence of blood markers from the embryo indicates that any putative bipotential B/EC precursors in the VBI were removed. Next we examined endothelial marker expression in embryos from which the VBI had been excised. Multiple markers were examined in the event that the VBI contributed a specific subpopulation of ECs to the embryonic vasculature. Endothelial marker expression appeared unchanged relative to controls for every marker assayed (Figure 1L-W). These experiments demonstrate that the Xenopus VBI is required for development of blood but contributes at most a small proportion of cells to the embryonic endothelium. Assay of the explant tissue at tailbud stage showed strong expression of blood differentiation markers as expected (Figure 1X), but we were surprised to find that expression of EC differentiation markers was undetectable (Figure 1Y). To control for the possibility that EC precursors migrated from the VBI earlier during development, we carried out excisions at st12, soon after B/EC markers are first expressed,4,21 and observed identical results to the st17 studies (supplemental Figure 2). All subsequent manipulations were carried out at st17, which is the time reported to have the greatest overlap of B/EC markers.4,5
VBI excision causes a loss of blood markers, whereas EC markers appear unaltered. (A) Schematic of the VBI region excision and subsequent embryo culture. After culturing to tailbud, control and manipulated embryos were assayed by in situ hybridization using the probes indicated in the panels. All manipulated embryos are labeled as ΔVBI. (B-K) Excision of the VBI eliminates expression of hematopoietic markers in the embryo. (B′-G′) Ventral views of the blood island region. (L-W) Excision of the VBI produces no consistent alteration in the pattern or intensity of the embryonic endothelium. Proportion of embryos displaying the in situ pattern illustrated is presented at lower right of each panel. (X-Y) Explant tissues at equivalent of st34, stained for blood (hba1) and endothelial (aplnr) differentiation markers, respectively. Note the absence of positive staining for the endothelial marker. Scale bar is 100 μm.
VBI excision causes a loss of blood markers, whereas EC markers appear unaltered. (A) Schematic of the VBI region excision and subsequent embryo culture. After culturing to tailbud, control and manipulated embryos were assayed by in situ hybridization using the probes indicated in the panels. All manipulated embryos are labeled as ΔVBI. (B-K) Excision of the VBI eliminates expression of hematopoietic markers in the embryo. (B′-G′) Ventral views of the blood island region. (L-W) Excision of the VBI produces no consistent alteration in the pattern or intensity of the embryonic endothelium. Proportion of embryos displaying the in situ pattern illustrated is presented at lower right of each panel. (X-Y) Explant tissues at equivalent of st34, stained for blood (hba1) and endothelial (aplnr) differentiation markers, respectively. Note the absence of positive staining for the endothelial marker. Scale bar is 100 μm.
No detectable enrichment of definitive endothelial markers in VBI explants
If B/EC precursors within the VBI are indeed a source of both hematopoietic cells and ECs, then culture of explanted VBIs in isolation should result in detection of cells expressing EC markers. Because expression of EC markers was undetectable by in situ hybridization (Figure 1Y; supplemental Figure 1), we used quantitative real-time polymerase chain reaction (qPCR) as a more sensitive assay. As a baseline control for these VBI explant studies, tissue was isolated from the VP region of the embryo, which normally produces very few ECs.14,19 VBI or VP regions were excised at st17 (supplemental Figure 3A). A fraction of the explants were immediately processed for qPCR analysis of B/EC precursor markers, whereas the remainder continued to develop until the tailbud stage. As expected, st17 VBI explants showed strong enrichment for the B/EC precursor markers etv2, erg, fli1, kdr, and tal1 compared with VP explants (supplemental Figure 3B).
Explants were cultured until the tailbud stage, with a proportion of the samples grown in the presence of VEGF, to stimulate/maintain the development of any EC precursors.15,23 Activity of the VEGF construction was demonstrated experimentally (supplemental Figure 4). VBI explants expressed high levels of erythroid differentiation markers relative to VP explants (supplemental Figure 3C), consistent with their known lineage potential, and the presence of VEGF down-regulated erythroid marker expression, in agreement with previous studies.24,25 In contrast, analysis of a range of EC differentiation markers showed no appreciable enrichment in the VBI explants relative to the negative control VP explants (supplemental Figure 3D). The presence of active VEGF did not result in up-regulation of endothelial marker expression. Consistent with our previous results (Figure 1Y), this experiment suggests that few ECs are produced in the VBI.
Most cells migrating from the VBI are myeloid
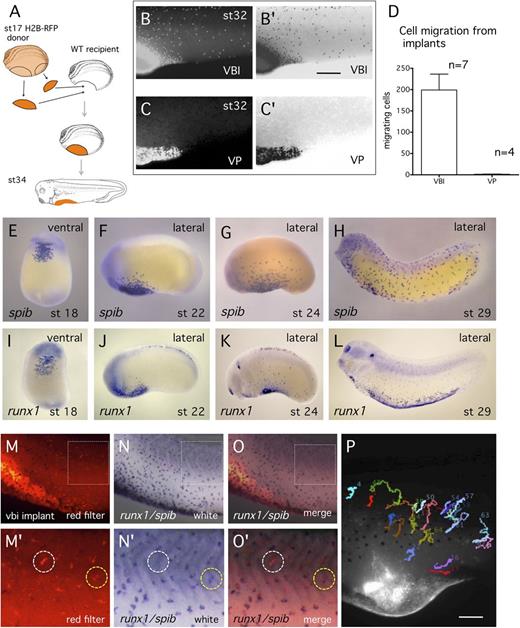
It is possible that excision of VBI tissue interfered with normal endothelial differentiation, and therefore we examined development of possible VBI-derived ECs in whole embryos. Because embryonic angioblasts are reported to be migratory,26 we reasoned that precursors might migrate from the VBI and differentiate elsewhere. To examine this possibility, fluorescent labeling was used to lineage trace cells of the VBI in the embryo. At st17, the VBI of host embryos was removed and replaced with the VBI of donors expressing fluorescent tracer (H2B-RFP) (Figure 2A). When transplanted embryos were observed by time-lapse imaging, migration of cells from the VBI was first observed at st20 to st21 and then in increasing numbers during subsequent development (Figure 2B; supplemental Video 1). Long-distance migration of cells is not a general property of embryonic tissue, because implantation of labeled VP tissue into the VBI region resulted in almost no migratory activity (Figure 2C-D). Both the time of emigration and the morphology of the migratory cells is consistent with embryonic myeloid cells22 (Figure 2E-L), and labeled cells migrated toward sites of injury on the epidermis, consistent with a myeloid phenotype (data not shown). When embryos were allowed to develop past the initiation of the heartbeat, labeled erythroid cells were observed circulating within embryonic vessels (data not shown).
The majority of cells migrating from the VBI are myeloid. (A) Schematic of transplant of lineage labeled VBI tissue to unlabeled recipient embryo. (B-C) Red light images showing migration of lineage labeled cells from the VBI implant and VP implant, respectively. (B′-C′) Image reversal of B and C to enhance visualization of labeled cells. (D) Quantitation of labeled cells per embryo migrating from the VBI and VP implants at early tailbud (st24). (E-L) In situ hybridization detection of myeloid cells using spib and runx1 probes. The pattern of distribution and time of emigration from the VBI of lineage labeled cells closely resembles the spib/runx1+ population. (M-O′) Myeloid cells in lineage-traced embryos were detected by double in situ hybridization with combined runx1 and spib probes (blue cells). Cells migrating from the VBI were detected by red filter imaging. Higher magnification of boxed region is shown in M′-O′. Note that most lineage-traced cells were positive for the myeloid probes (yellow circle), whereas a small proportion was not (white circle). (P) Single frame from supplemental Video 2 of lineage labeled embryo from st36-42. Cells paths were tracked using MTrackJ.18 All labeled cells were observed to migrate over time, consistent with blood, but not EC behavior.
The majority of cells migrating from the VBI are myeloid. (A) Schematic of transplant of lineage labeled VBI tissue to unlabeled recipient embryo. (B-C) Red light images showing migration of lineage labeled cells from the VBI implant and VP implant, respectively. (B′-C′) Image reversal of B and C to enhance visualization of labeled cells. (D) Quantitation of labeled cells per embryo migrating from the VBI and VP implants at early tailbud (st24). (E-L) In situ hybridization detection of myeloid cells using spib and runx1 probes. The pattern of distribution and time of emigration from the VBI of lineage labeled cells closely resembles the spib/runx1+ population. (M-O′) Myeloid cells in lineage-traced embryos were detected by double in situ hybridization with combined runx1 and spib probes (blue cells). Cells migrating from the VBI were detected by red filter imaging. Higher magnification of boxed region is shown in M′-O′. Note that most lineage-traced cells were positive for the myeloid probes (yellow circle), whereas a small proportion was not (white circle). (P) Single frame from supplemental Video 2 of lineage labeled embryo from st36-42. Cells paths were tracked using MTrackJ.18 All labeled cells were observed to migrate over time, consistent with blood, but not EC behavior.
To identify myeloid cells using molecular markers, we carried out VBI transplants using red Dextran labeled donor tissue, because this fluorescent dye can withstand fixation. When transplanted embryos were assayed by double in situ hybridization using the myeloid markers spib and runx1 (Figure 2N-N′), we observed that ∼88% of Dextran-labeled cells colocalized with myeloid markers (290/328 cells, n = 4 embryos; Figure 2M-O′). However, a significant number of cells were not detected using the myeloid probes (white ring in Fig. 2O′). These cells are candidates for VBI-derived ECs but alternatively could be hematopoietic cells that are not expressing spib or runx1.
When angioblasts differentiate into ECs, they express cell adhesion molecules, cease migration, and assemble into tubular vessels.26,27 To determine whether any of the labeled VBI-derived cells were postmigratory (consistent with a differentiated endothelial phenotype), embryos were observed by time-lapse microscopy for ∼7 hours after initiation of heartbeat. Fluorescently labeled cells were then manually tracked to assess migration (Figure 2P; supplemental Video 2). Of >200 cells examined, all displayed migratory behavior, consistent with a myeloid, but not an endothelial, phenotype. These results lead us to conclude that any contribution of VBI-derived cells to the embryonic vasculature must be rather small. It also suggests that most of the cells that were fluorescently labeled, but not positive for spib or runx1, were immune system cells and not ECs.
Using molecular markers, no VBI-derived ECs were detected
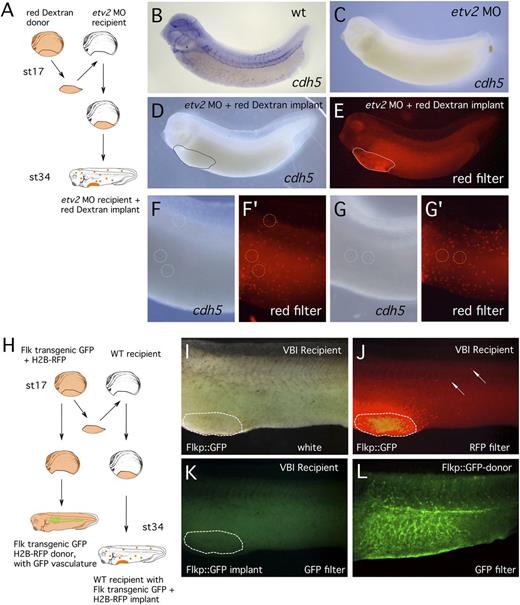
Our results show that if any VBI derived ECs exist, they are not abundant. Therefore, we carried out 2 additional studies in an attempt to detect these rare cells. First, we asked whether transplantation of wild-type VBI (containing B/EC precursors) could replenish embryos in which the molecular pathway controlling EC development was blocked by depletion of the critical transcription factor Etv2. Previous studies have shown that loss of Etv2 eliminates EC specification and marker expression.17,28,29 Therefore, we transplanted wild-type red Dextran-labeled VBI regions into host embryos injected with etv2 MO (Figure 3A). After developing to the tailbud stage, experimental embryos were assayed by in situ hybridization for expression of the EC differentiation marker cdh5. Because Etv2 activity is depleted, the only ECs detected in the host embryo should be red Dextran-labeled cells derived from the wild-type VBI. First, we confirmed that injection of the etv2 MO almost completely eliminated detectable cdh5 expression (Figure 3B-C). In VBI implant embryos (Figure 3D-G′), red-labeled cells from the VBI were present in normal numbers distributed throughout the recipient embryos (Figure 3F′,G′). However, the rare cdh5-positive cells in recipient embryos (circled regions in Figure 3F-G) were never Dextran labeled (n = 8 embryos), indicating that they were not VBI derived.
No VBI-derived cells were detected in the embryonic endothelium. (A) Schematic outline of Etv2 knockdown experiment. (B-C) In situ hybridization detection of endothelial differentiation marker (cdh5) in (B) control and (C) Etv2 knockdown embryo, showing elimination of marker gene expression. (D) Experimental embryo visualized after in situ hybridization for EC marker (cdh5). (E) The same embryo viewed under a red filter and showing abundance of lineage traced cells migrating from the implant. (F-G) White light images of 2 independent cdh5-stained embryos showing near absence of detectable ECs. Rare examples of staining are circled. (F′-G′) Red filter images of the same embryos shown in F and G. None of the red Dextran lineage-traced cells correspond precisely to the position of the in situ stain (circles). (H) Schematic outline of transgenic endothelial marker experiment, using Flkp::GFP donor tissue. (I-K) White light, red filter, and GFP filter images of the same implanted embryo. Although (J) abundant cells have migrated from the VBI implant, (K) none of these cells are expressing GFP marker consistent with ECs. (L) Transgenic embryo that contributed the VBI implant, showing prominent endothelial GFP expression.
No VBI-derived cells were detected in the embryonic endothelium. (A) Schematic outline of Etv2 knockdown experiment. (B-C) In situ hybridization detection of endothelial differentiation marker (cdh5) in (B) control and (C) Etv2 knockdown embryo, showing elimination of marker gene expression. (D) Experimental embryo visualized after in situ hybridization for EC marker (cdh5). (E) The same embryo viewed under a red filter and showing abundance of lineage traced cells migrating from the implant. (F-G) White light images of 2 independent cdh5-stained embryos showing near absence of detectable ECs. Rare examples of staining are circled. (F′-G′) Red filter images of the same embryos shown in F and G. None of the red Dextran lineage-traced cells correspond precisely to the position of the in situ stain (circles). (H) Schematic outline of transgenic endothelial marker experiment, using Flkp::GFP donor tissue. (I-K) White light, red filter, and GFP filter images of the same implanted embryo. Although (J) abundant cells have migrated from the VBI implant, (K) none of these cells are expressing GFP marker consistent with ECs. (L) Transgenic embryo that contributed the VBI implant, showing prominent endothelial GFP expression.
Second, to rule out the possibility that the Etv2 depletion might somehow affect migration or survival of wild-type derived EC within the host embryo, we carried out related studies in a wild-type host environment (Figure 3H). Briefly, Flk1::EGFP transgenic donor embryos19 were injected with lineage tracer (H2B-RFP), and at st17, the VBI region was transplanted into wild-type host embryos. If cells from the VBI contribute to the embryonic vasculature, then RFP-labeled, GFP-positive cells will be present in the endothelium of the host embryo during later development. As expected, numerous RFP-labeled cells were visible in host embryos (Figure 3J); however, unlike the transgenic donor embryo, which showed GFP expression throughout the developing vasculature (Figure 3L), no GFP/RFP double-labeled cells were detected in any of the recipient embryos (Figure 3K; n = 6). Because ∼400 red-labeled VBI-derived cells were counted in each of the host embryos, these results show that VBI-derived ECs are extremely rare and possibly nonexistent in the Xenopus embryo.
BMP signaling inhibits EC differentiation in the VBI
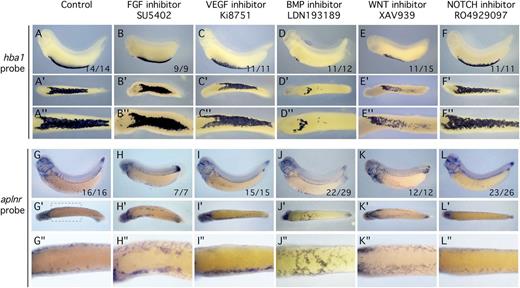
Previous studies have implicated the fibroblast growth factor, VEGF, BMP, WNT, and NOTCH signaling pathways in regulating the relative abundance of hematopoietic vs ECs in the embryo.4,30-35 We tested whether these signaling pathways might regulate the blood/endothelial fate decision in the Xenopus VBI (Figure 4). In all cases, embryos were treated continuously from postgastrulation until tailbud using small molecule inhibitors specific for individual pathways. The effectiveness of the inhibitors was demonstrated experimentally (supplemental Figure 5). Treatment was commenced after gastrulation to allow completion of primary axial patterning of the embryo before signaling pathways were inhibited. Examination of the globin (hba1) in situ pattern showed that expression was similar to controls for all inhibitors except those of the BMP and WNT pathways, which caused a marked reduction in globin staining (Figure 4D-E″). When endothelial markers were analyzed, only BMP inhibition consistently resulted in ectopic endothelial expression in the VBI region (Figure 4J-J″). Previous studies have shown that WNT modulates BMP in the Xenopus VBI,36 suggesting that the observed effects are mediated through the BMP pathway.
Growth factor inhibitor screen shows BMP signaling is necessary for erythroid development and inhibitory for EC specification. All embryos were treated with inhibitor starting at st13 and assayed by in situ hybridization at st32. Blood differentiation marker was hba1 and the EC differentiation marker was aplnr. Inhibitors are indicated at the top of each column. (A,G) Control embryo treated with dimethylsulfoxide carrier. (B,H) Inhibition of the FGF signaling pathway using the specific inhibitor SU5402. (C,I) Inhibition of the VEGF signaling pathway using the specific inhibitor Ki8751. (D,J) Inhibition of the BMP signaling pathway using the specific inhibitor LDN193189. (E,K) Inhibition of the WNT signaling pathway using the specific inhibitor XAV939. (F,L) Inhibition of the NOTCH signaling pathway using the specific inhibitor RO4929097. For each treatment and probe, the same embryo is shown in a lateral, ventral (′), and magnified ventral (″) view. Of the experimental treatments, inhibition of BMP signaling had the most dramatic effect on reducing blood differentiation while enabling EC differentiation. Numbers at lower right of the lateral view panel indicate the number of embryos displaying the illustrated phenotype.
Growth factor inhibitor screen shows BMP signaling is necessary for erythroid development and inhibitory for EC specification. All embryos were treated with inhibitor starting at st13 and assayed by in situ hybridization at st32. Blood differentiation marker was hba1 and the EC differentiation marker was aplnr. Inhibitors are indicated at the top of each column. (A,G) Control embryo treated with dimethylsulfoxide carrier. (B,H) Inhibition of the FGF signaling pathway using the specific inhibitor SU5402. (C,I) Inhibition of the VEGF signaling pathway using the specific inhibitor Ki8751. (D,J) Inhibition of the BMP signaling pathway using the specific inhibitor LDN193189. (E,K) Inhibition of the WNT signaling pathway using the specific inhibitor XAV939. (F,L) Inhibition of the NOTCH signaling pathway using the specific inhibitor RO4929097. For each treatment and probe, the same embryo is shown in a lateral, ventral (′), and magnified ventral (″) view. Of the experimental treatments, inhibition of BMP signaling had the most dramatic effect on reducing blood differentiation while enabling EC differentiation. Numbers at lower right of the lateral view panel indicate the number of embryos displaying the illustrated phenotype.
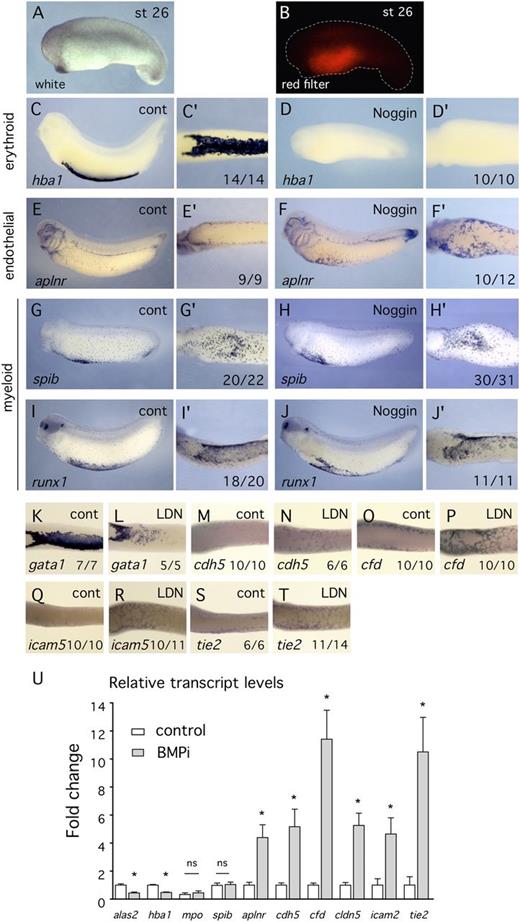
Because small molecule inhibitors can exhibit off-target effects or nonspecific toxicity, we tested whether Noggin, a protein inhibitor of BMP signaling,37 and another small molecule BMP inhibitor, DMH1, exhibited similar effects on blood and endothelial marker expression. The results confirm that inhibition of BMP signaling increased endothelial marker expression at the expense of blood markers (Figure 5; supplemental Figure 6). Targeted inhibition of BMP signaling in the VBI using Noggin resulted in complete elimination of globin expression (Figure 5D-D′) and ectopic EC marker expression (Figure 5F-F′). Noggin was probably more effective than small molecule inhibitors because it was present throughout early development while the small molecules were added only after gastrulation. In addition to erythroid and endothelial markers, we examined the effect of inhibition of BMP signaling on the myeloid markers mpo, runx1, and spib. In situ hybridization analysis and quantitation of cell numbers showed that myeloid development was unaltered by inhibition of BMP (Figures 5G-J′ and 6H-M′′; supplemental Figure 7), indicating that the precursors of the erythroid and myeloid lineages respond independently to BMP signaling. Furthermore, the observation that myeloid development was normal indicates that patterning of extreme ventral tissues was not disrupted by inhibition of BMP signaling.
Use of the BMP inhibitor protein, Noggin, confirms that BMP signaling is required for erythroid specification and inhibits EC differentiation. (A-B) White light and red filter images, respectively, of the same embryo showing targeting of Noggin expression to the VBI region. The reporter for protein distribution is H2B-RFP. (C-T) All embryos were assayed by in situ hybridization at st32 using probes indicated at lower left of each panel. Number of embryos showing illustrated effect is at lower right. (C-J′) Embryos treated with Noggin. An enlarged ventral view (′) is provided for each embryo. (C-F′) Inhibition of BMP signaling using Noggin results in elimination of blood marker expression (hba1) and appearance of EC marker expression (aplnr) in the ventral region. (G-J′) Inhibition of BMP signaling using Noggin has no effect on expression of myeloid markers (spib and runx1). (K-T) Ventral view of controls and embryos treated with LDN193189. Erythroid marker expression assayed with gata1 is decreased, whereas expression of endothelial differentiation markers is increased. (U) qPCR analysis of VBI explant tissue treated with the BMP inhibitor, LDN193189, from st13 to st32. Compared with controls, erythroid marker expression is decreased, myeloid marker expression is unchanged, and endothelial marker expression is increased.
Use of the BMP inhibitor protein, Noggin, confirms that BMP signaling is required for erythroid specification and inhibits EC differentiation. (A-B) White light and red filter images, respectively, of the same embryo showing targeting of Noggin expression to the VBI region. The reporter for protein distribution is H2B-RFP. (C-T) All embryos were assayed by in situ hybridization at st32 using probes indicated at lower left of each panel. Number of embryos showing illustrated effect is at lower right. (C-J′) Embryos treated with Noggin. An enlarged ventral view (′) is provided for each embryo. (C-F′) Inhibition of BMP signaling using Noggin results in elimination of blood marker expression (hba1) and appearance of EC marker expression (aplnr) in the ventral region. (G-J′) Inhibition of BMP signaling using Noggin has no effect on expression of myeloid markers (spib and runx1). (K-T) Ventral view of controls and embryos treated with LDN193189. Erythroid marker expression assayed with gata1 is decreased, whereas expression of endothelial differentiation markers is increased. (U) qPCR analysis of VBI explant tissue treated with the BMP inhibitor, LDN193189, from st13 to st32. Compared with controls, erythroid marker expression is decreased, myeloid marker expression is unchanged, and endothelial marker expression is increased.
Examination of additional EC differentiation markers in BMP-inhibited embryos confirmed increased expression of EC genes in the VBI region (Figure 5M-T). These results were validated by qPCR when VBI explants treated with BMP inhibitor were assayed for blood and EC marker expression (Figure 5U).
The appearance of ECs in the VBI domain can be explained in at least 2 different ways. First, multipotent precursors may have changed fate from the erythroid to the endothelial lineage. Second, inhibition of BMP signaling may have eliminated the blood lineage and, perhaps independently, allowed lateral ECs to migrate into the ventral domain. To distinguish between these possibilities, we first made VBI explants from unmanipulated postgastrula embryos, treated the explants with BMP inhibitor or control medium, and assayed for EC markers at the tailbud stage (supplemental Figure 8). Because the explanted tissues were isolated from the embryo, it would not be possible for angioblasts from more dorsal lateral regions to migrate into the VBI region. As in intact embryos, BMP inhibition resulted in reduction or elimination of erythroid marker expression (supplemental Figure 8A-B), whereas robust expression of endothelial markers was observed within the explanted tissue (supplemental Figure 8C-D). We conclude that ECs are derived from within the explanted VBI tissue and not from the lateral plexus.
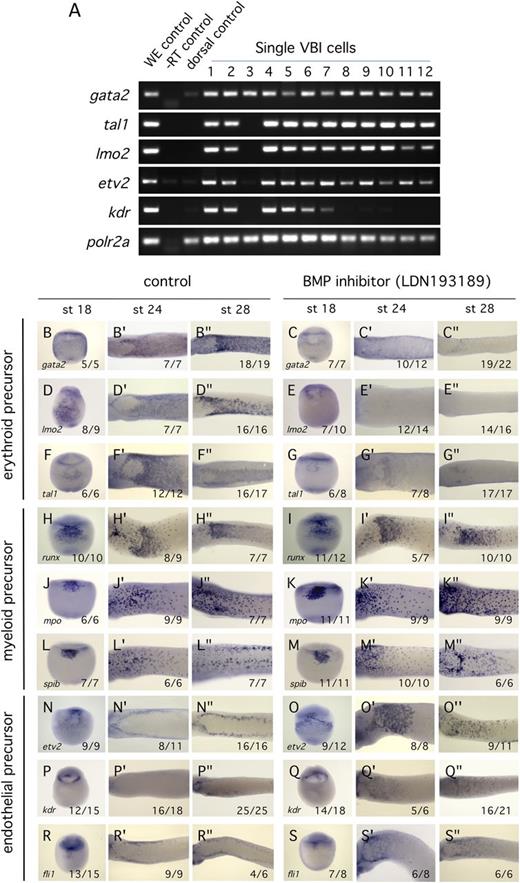
Second, we confirmed that erythroid and EC precursor markers were indeed expressed in the same cells. Single-cell PCR analysis of cells isolated from the mesodermal layer of the VBI confirmed that transcripts of the EC marker, kdr, were coexpressed with hematopoietic precursor markers (Figure 6A). Third, we carried out a time course analysis of B/EC precursor marker expression in response to inhibition of BMP signaling. In control embryos, VBI expression of the erythroid precursor markers gata2, lmo2, and tal1 increased as blood development progressed (Figure 6B,D,F), whereas expression of angioblast markers decreased (Figure 6N,P,R). In contrast, when embryos were treated with BMP inhibitor, expression of the erythroid markers rapidly decreased (Figure 6C,E,G), and expression of angioblast markers persisted (Figure 6O,Q,S). Expression of myeloid markers was unchanged by BMP inhibition (Figure 6H-M). These results indicate that expression of EC precursor markers was maintained in the precursor cell population and did not arise in a different cell population in response to inhibited BMP signaling.
Inhibition of BMP signaling results in reduction in expression of blood precursor markers and persistence of expression of endothelial precursor markers. (A) Single cell PCR analysis of precursor gene expression in st17 VBI. Individual cells were isolated from dissected VBI mesoderm layer and transcripts assayed by PCR. Whole embryo (WE) tissue was used as a positive control. A single cell from the dorsal region of the st17 embryo was used as a negative control for B/EC expression. Transcripts for RNA polymerase II, polr2a, were used as a reference standard. Method details can be found in supplemental Materials. Cell number 3 is probably an endodermal contaminant. Note that the EC precursor markers kdr and etv2 are coexpressed with hematopoietic precursor markers gata2, tal1, and lmo2. (B-S″) Time course of precursor marker expression in VBI. Treatment with BMP inhibitor commenced at st13. All embryos were assayed by in situ hybridization using the probe indicated. All views are ventral. Embryonic stages assayed are indicated at the top of each column. Erythroid precursor markers are gata2, lmo2, and tal1. Myeloid precursor markers are runx1, mpo, and spib. Endothelial markers are etv2, kdr, and fli1. Numbers at the lower right of each panel indicate the proportion of embryos displaying the illustrated phenotype.
Inhibition of BMP signaling results in reduction in expression of blood precursor markers and persistence of expression of endothelial precursor markers. (A) Single cell PCR analysis of precursor gene expression in st17 VBI. Individual cells were isolated from dissected VBI mesoderm layer and transcripts assayed by PCR. Whole embryo (WE) tissue was used as a positive control. A single cell from the dorsal region of the st17 embryo was used as a negative control for B/EC expression. Transcripts for RNA polymerase II, polr2a, were used as a reference standard. Method details can be found in supplemental Materials. Cell number 3 is probably an endodermal contaminant. Note that the EC precursor markers kdr and etv2 are coexpressed with hematopoietic precursor markers gata2, tal1, and lmo2. (B-S″) Time course of precursor marker expression in VBI. Treatment with BMP inhibitor commenced at st13. All embryos were assayed by in situ hybridization using the probe indicated. All views are ventral. Embryonic stages assayed are indicated at the top of each column. Erythroid precursor markers are gata2, lmo2, and tal1. Myeloid precursor markers are runx1, mpo, and spib. Endothelial markers are etv2, kdr, and fli1. Numbers at the lower right of each panel indicate the proportion of embryos displaying the illustrated phenotype.
Ectopic endothelial differentiation is enabled when the erythroid transcriptional program is disrupted
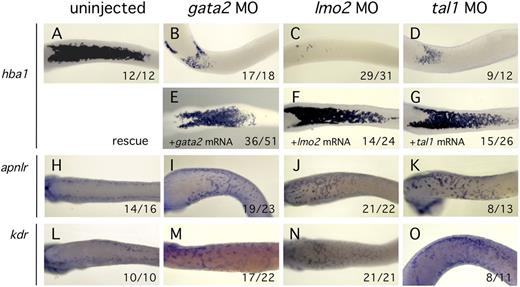
BMP signaling may have multiple different roles in modulation of cell fate or behavior in the blood island, but perhaps the most critical function is as an activator of erythroid differentiation.38,39 To test this proposition, we cell autonomously disrupted erythroid development by knockdown of critical blood lineage transcription factors in the context of normal BMP signaling. Previous studies have demonstrated that the transcription factors Gata2, Lmo2, and Tal1 are essential for erythroid development.16,40-42 MO-mediated depletion of each of these transcription factors resulted in reduced erythroid differentiation as expected (Figure 7B-D). Significantly we found that blocking blood development by knockdown of Gata2, Lmo2, or Tal1 was sufficient to induce robust expression of angioblast markers in the VBI region (Figure 7I-K,M-O). These results suggest that, during normal development, commitment to the erythroid lineage blocks EC fate. However, when development of the erythroid lineage is disrupted, differentiation of precursors to mature ECs is able to proceed.
Inhibition of the erythroid transcriptional program enables expression of endothelial markers. All embryos were assayed by in situ hybridization at st32. Ventral views are presented for all embryos. MO treatment is indicated at the top of each column. (A-D) Knockdown of Gata2, Lmo2, or Tal1 activity reduced erythroid differentiation marker expression (hba1) relative to controls. (E-G) Specificity of MO knockdown was demonstrated by efficient rescue after coinjection of MO plus the cognate mRNA. (H-O) Knockdown of Gata2, Lmo2, or Tal1 resulted in high level expression of EC markers (aplnr and kdr) at the ventral midline compared with controls. Numbers at the lower right of each panel indicate the proportion of embryos displaying the illustrated phenotype.
Inhibition of the erythroid transcriptional program enables expression of endothelial markers. All embryos were assayed by in situ hybridization at st32. Ventral views are presented for all embryos. MO treatment is indicated at the top of each column. (A-D) Knockdown of Gata2, Lmo2, or Tal1 activity reduced erythroid differentiation marker expression (hba1) relative to controls. (E-G) Specificity of MO knockdown was demonstrated by efficient rescue after coinjection of MO plus the cognate mRNA. (H-O) Knockdown of Gata2, Lmo2, or Tal1 resulted in high level expression of EC markers (aplnr and kdr) at the ventral midline compared with controls. Numbers at the lower right of each panel indicate the proportion of embryos displaying the illustrated phenotype.
Discussion
Despite elegant experiments in mice, chicks, and zebrafish, in vivo existence of the hemangioblast remains controversial.10-13 Starting at the late gastrula stage, markers of both hematopoietic and endothelial precursors are expressed in the VBI of Xenopus.4,5,17,21 Coexpression of these markers raises the possibility that the blood island might contain cells with hemangioblast properties. Although hematopoiesis within the VBI is well established, development of ECs from VBI precursors has not previously been investigated. In the studies reported here, no VBI-derived ECs were detected (Figures 1-3; supplemental Figures 2-3). Because the classically defined hemangioblast gives rise to both endothelial and hematopoietic progeny, our results infer that hemangioblasts are not present in the Xenopus blood island during normal development.
During early postgastrula development, angioblast markers are expressed at high levels in the VBI, but expression is lost ∼12 hours later by early tailbud stages, and these cells never form blood vessels. Over this same period, expression of hematopoietic markers expands. When we examined the role of different growth factor signaling pathways in this process, we found that BMP was essential for maintenance of the erythroid program (Figures 4-6; supplemental Figure 6). When BMP signaling was compromised using either small molecule or protein reagents, markers of the erythroid lineage disappeared while angioblast markers persisted and EC differentiation markers appeared. Nonerythroid blood lineages appeared to be unaffected by inhibition of BMP signaling (Figures 5G-J′ and 6H-M′′; supplemental Figures 7 and 8G-H). The critical role of BMP signaling in embryonic hematopoietic development is well established,16,33,38,39 but the reciprocal relationship between erythroid and endothelial differentiation in the blood islands has not been reported previously. Similar to the original hemangioblast hypothesis, our work using BMP inhibitors indicates a remarkably close relationship between the endothelial and blood lineages, particularly the erythroid lineage. However, although VBI cells double positive for B/EC precursor markers have a latent potential to develop toward either blood or endothelial lineages, we find that only blood cells are derived from the VBI precursor population during normal development, indicating that these cells are not classical hemangioblasts.
Although growth factor signaling could play multiple instructional or inhibitory roles in the VBI, we focused on the recognized role of BMP in promoting blood development.43 We reasoned that activation of the blood program by BMP might prevent further development of the precursor population toward endothelial differentiation. A very similar model has been proposed by Nishikawa, who hypothesized that, within a precursor population, specification of the blood lineage might obviate the endothelial program.44 We directly tested this proposal by reducing the activity of 3 individual components of the erythroid transcriptional machinery: Gata2, Lmo2, and Tal1 (Figure 6). In each case, erythroid differentiation was severely reduced, with a concomitant appearance of endothelial differentiation markers. The lineage fate switch occurred in the context of normal BMP signaling, because it has previously been shown that disruption of blood development does not alter BMP expression.16 In agreement with the Nishikawa prediction,44 these results strongly suggest that commitment to the erythroid lineage eliminates the potential for endothelial differentiation in the precursor population. These results also raise the possibility that transcription factors required for activation of the erythroid transcriptional pathway might simultaneously suppress the endothelial transcriptional program in the precursor population.45
What population of cells in the amniote embryo is the functional equivalent of the early postgastrula blood island of Xenopus? Lineage tracing studies in mice have shown that cells normally giving rise to extra-embryonic blood and endothelium occupy distinct locations in the primitive streak.46 However, Flk+ cells isolated from regions of the early and mid-streak can develop into endothelial and hematopoietic cells in clonal culture.7,9 We suggest that cells in the early Xenopus blood island are equivalent to the previously described Flk+ precursor population in the mouse streak. This raises the possibility that cells ingressing through the amniote streak at slightly different times encounter different BMP signaling activity. Cells of the mouse early streak population may encounter BMP ligand38 and differentiate into extra-embryonic blood. If cells that ingress at mid-streak do not receive BMP signals, they will develop as extra-embryonic endothelium.46
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Parker Antin for the use of the time-lapse imaging microscope, Cristy Lewis for dorsal aorta images, Aaron Zorn and Ondine Cleaver for comments on the manuscript, and Sarah Appleby for help with in situ hybridizations.
C.T.M. is supported by National Institutes of Health, National Institute of General Medical Sciences training grant T32 GM08659 and the Philanthropic Educational Outreach Scholar award. P.A.K. is the Allan C. Hudson and Helen Lovaas Endowed Professor of the Sarver Heart Center at the University of Arizona College of Medicine. This work was supported by the Sarver Heart Center and by Heart, Lung, and Blood Institute grant HL093694.
Authorship
Contribution: C.T.M. designed the study, performed experiments, analyzed data, and wrote the manuscript; and P.A.K. designed the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paul A. Krieg, Department of Cellular and Molecular Medicine, University of Arizona College of Medicine, 1656 E Mabel St, MRB311, Tucson, AZ 85724; e-mail: pkrieg@e-mail.arizona.edu.