In this issue of Blood, Zimmerman and colleagues demonstrate that the tyrosine kinase inhibitor (TKI) crenolanib effectively suppresses growth of leukemic cells harboring both FLT3-ITD and FLT3-TKD mutations, the latter of which are increasingly seen to emerge as resistant mutations after FMS-like tyrosine kinase 3 (FLT3) inhibitor therapy.1
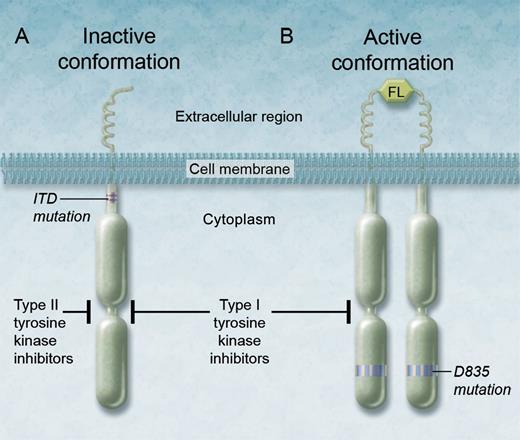
Binding of the ITD-altered FLT3 tyrosine kinase receptor by type I and type II TKIs. (A) Type II inhibitors are thought to bind the inactive conformation of the FLT3 receptor tyrosine kinase and in this manner potently inhibit the ITD-altered FLT3 tyrosine kinase, but they have limited activity against secondary point mutations such as D835, because (B) D835 point mutations are thought to destabilize the inactive conformation of the FLT3 kinase in favor of the active conformation. Therefore, type I inhibitors, such as crenolanib, which can also bind the active conformation of the enzyme, can effectively inhibit the FLT3 kinase altered by the D835 mutation. FL, FLT3 ligand. Professional illustration by Alice Y. Chen.
Binding of the ITD-altered FLT3 tyrosine kinase receptor by type I and type II TKIs. (A) Type II inhibitors are thought to bind the inactive conformation of the FLT3 receptor tyrosine kinase and in this manner potently inhibit the ITD-altered FLT3 tyrosine kinase, but they have limited activity against secondary point mutations such as D835, because (B) D835 point mutations are thought to destabilize the inactive conformation of the FLT3 kinase in favor of the active conformation. Therefore, type I inhibitors, such as crenolanib, which can also bind the active conformation of the enzyme, can effectively inhibit the FLT3 kinase altered by the D835 mutation. FL, FLT3 ligand. Professional illustration by Alice Y. Chen.
Despite incremental improvements in outcomes in the last three decades, acute myeloid leukemia (AML) continues to be a clinically challenging and frequently lethal hematologic malignancy. Internal tandem duplication (ITD) mutations of the FLT3 gene are found in approximately one-quarter of those diagnosed with AML. ITD alterations affect the juxtamembrane domain of the receptor.2,3 Point mutations within the activation loop of the tyrosine kinase domain (TKD) of FLT3, with the majority at the D835 residue, are less frequent and present in ∼8% of patients.4,5 The prognostic impact of FLT3 mutations has been the subject of significant clinical study in the last decade, which has in turn led to their incorporation as standard assays performed at diagnosis for AML. The ITD mutations in particular have been associated with a poor prognosis, predominantly related to a marked propensity for disease relapse.5,6 This is related to the molecular impact of the mutations, which render the receptor tyrosine kinase constitutively active, in turn triggering multiple signaling cascades such as those involving the STAT5, RAS and MEK, as well as the PI3K/AKT pathways. This results in suppression of cellular apoptosis and differentiation, and dysregulated leukemic proliferation.7 Intriguingly, FLT3-TKD mutations have generally not been associated with a similar degree of negative prognosis as ITD mutations in clinical studies.5
There has thus been a strong rationale to develop effective TKIs of FLT3 as targeted therapy for AML patients, and in the last decade, a variety of inhibitor have been studied, with several currently in various phases of clinical investigation. To date, the relative nonselectivity of some of these agents, and suboptimal pharmacokinetics associated with others, have led to unimpressive results in their clinical development. In recent years, more selective and/or potent FLT3 inhibitors, such as quizartinib and sorafenib, have been associated with more profound responses, including bone marrow remissions.2 Nevertheless, despite the promise of FLT3 as a therapeutic target, significant challenges remain.
Among these challenges is the development of therapeutic resistance to FLT3 inhibitors with concurrent disease progression, which has been increasingly noted as these agents have been used off-protocol or on clinical study in recent years.8 Investigators have found that a common mechanism of resistance following use of FLT3 TKI therapy in ITD disease is the development of kinase domain activating point mutations, a particularly difficult obstacle in the management of individual patients with advanced AML, and in the over-arching goal of developing effective and durable therapies for FLT3-mutant AML.
In the current edition of Blood, Zimmerman and colleagues attempt to investigate the feasibility of effective inhibition of FLT3 through use of crenolanib, a TKI with suspected type I properties. Type I TKIs can bind the active or inactive conformations of receptor tyrosine kinases, unlike type II inhibitors, which only bind the inactive form (see figure). Point mutations within the kinase domain are thought to destabilize the inactive conformation of FLT3. With either type I or type II inhibition, downstream signaling of the FLT3 receptor is aborted, leading to suppression of signaling through key pathways and thereby promoting differentiation and apoptosis of leukemic cells. Most FLT3 inhibitors investigated to date, with predominantly type II properties, have minimal activity against FLT3-TKD AML. This in turn selects for a persistence of TKD clones or emergence of new ones, which in part explains the development of therapeutic resistance increasingly seen after FLT3 inhibitor therapy.
In this study, the investigators report several important observations. First, crenolanib was a potent inhibitor of both FLT3-ITD and FLT3-TKD mutations. It was noted to effectively suppress FLT3-ITD leukemic cells, as demonstrated in ITD cell lines, such as MOLM-13 and MV4-11, as well as in a xenograft model. They also found that cells expressing either point mutations alone or combined with ITD mutations were more responsive to crenolanib than to the type II FLT3 inhibitor sorafenib. It is important to note that the profound efficacy of crenolanib against FLT-D835 and FLT3-ITD mutant AML has been previously reported by other groups,9,10 although the current report expands and confirms these observations through study of both in vitro and in vivo models. They further observed that simultaneous treatment with sorafenib and crenolanib led to even greater anti-leukemic activity, suggesting that dual type I and type II inhibition may increase efficacy by concurrently targeting the active and inactive kinase conformations of FLT3. Simultaneous inhibition may also suppress the outgrowth or emergence of resistant disease, which frequently demonstrate TKD mutations. Of note, the efficacy of crenolanib was also evaluated in a limited number of TKI-resistant primary samples, which had acquired D835H/Y mutations, and found to be more profound than that of sorafenib.
These findings, along with those reported by others, suggest that crenolanib may have a future role as a clinically active agent against AML with activation loop mutations, such as those affecting D835. Whether crenolanib can be incorporated into frontline therapeutic approaches for patients with FLT3-ITD and/or TKD mutations or whether it will best serve in recapturing response in patients who have developed secondary activating point mutations requires further study. There are currently multiple phase II studies evaluating crenolanib in the relapsed and refractory setting. In addition, combined type I and type II TKI therapy is an intriguing concept to help enhance efficacy and perhaps suppress the emergence of therapeutic resistance. However, given the individual toxicities and pharmacokinetic properties of such agents, optimizing the tolerability of combined TKI therapy, such as with sorafenib and crenolanib, may be challenging. In summary, the recent introduction of potent and targeted agents, such as quizartinib and crenolanib, for FLT3-mutant disease increases hope for a new era of effective and durable therapies for this frequently lethal subtype of AML.
Conflict-of-interest disclosure: The author declares no competing financial interests.