Abstract
Myelodysplastic syndrome (MDS) is characterized by dysplastic and ineffective hematopoiesis, peripheral blood cytopenias and a risk of leukemic transformation. Most MDS patients eventually require red blood cell (RBC) transfusions for anemia and consequently develop iron overload. Excess free iron in cells catalyzes generation of reactive oxygen species (ROS) especially hydroxyl radical that cause oxidative DNA damage including formation of mutagenic 8-hydroxy-2'-deoxyguanosine (8-OHdG) and DNA double strand breaks. However, it is unclear whether iron-mediated oxidative stress affects the pathophysiology of MDS.
This study included MDS patients who visited our university hospital and affiliated hospitals (n=43). Among them, 13 patients received iron chelation therapy when their serum ferritin (SF) level was greater than 1000 ng/mL or they required more than 20 RBC transfusions (or 100 mL/kg of RBC). We analyzed hemoglobin, SF level and chromosomal abnormality and 8-OHdG levels in peripheral blood mononuclear cells (PBMC) obtained from MDS patients before and after iron chelator, deferasirox administration.
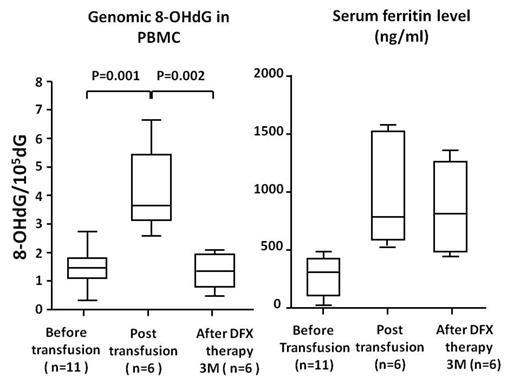
We showed that reactive oxygen species (ROS) in hematopoietic cells was higher in MDS patients with high SF level. Furthermore, the 8-OHdG levels in MDS patients were significantly higher than those in healthy volunteers and the 8-OHdG levels were positively correlated with SF (p=0.003, r=0.495) and chromosomal abnormalities (p=0.019). Importantly, the 8-OHdG levels in PBMC and CD34+ cells of MDS patients dramatically decreased 3 month after deferasirox administration (the 8-OHdG levels in PBMC, 3.374±78, p<0.05) although SF level were not reduced within 3 month (Figure). However, SF level decreased to 500-600 ng/mL after 36 months of iron chelation therapy, 8-OHdG is a sensitive marker of cellular iron chelation therapy by deferasirox which readily passes through the plasma membrane. When patients were divided into the no Iron chelation group (n=10) and high Iron chelation group (n=13), the survival time was significantly longer in the latter group than the former group (P=0.046) although International prognostic scoring system (IPSS) was not significantly difference between both groups. Further, 50% of patients without iron chelation group developed into leukemia, while no patients did progress into leukemia during 36 month.
These results indicated that cellular excess iron could contribute to the pathophysiology of MDS and iron chelation therapy could improve the oxidative DNA damage in MDS patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal