Abstract
Voriconazole is a common antifungal medication used in allogeneic hematopoietic stem cell transplantation (allo-HSCT) patients. In solid organ transplantation, multiple studies have associated the use of voriconazole with the development of squamous cell carcinoma (SCC) post-transplant, but its association with SCC in allo-HSCT patients is unknown. We sought to determine this association.
After IRB approval, Mayo Clinic’s transplant database (2007-2012) was accessed and electronic charts of allo-HSCT patients were retrospectively reviewed. Voriconazole exposure was defined as exposure to voriconazole at any time during treatment of primary disease, prior to or following HSCT. Cumulative voriconazole exposure was defined as total days of voriconazole use following HSCT; days were not required to be consecutive. Two time-dependent voriconazole exposure variables were defined: (1) history of voriconazole exposure (yes/no) over time, and (2) cumulative days on voriconazole over time.
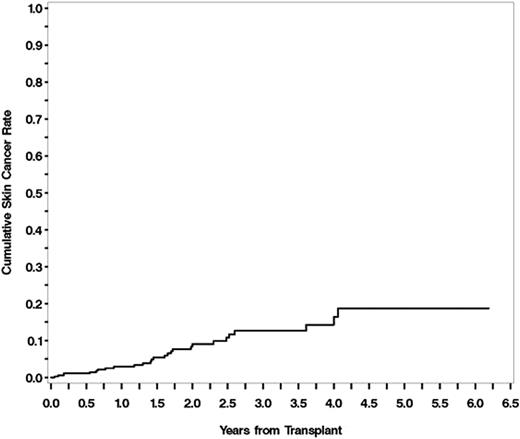
404 patients underwent allo-HSCT during this timeframe, and 381 patients (table 1) were included in the final analysis. 23 patients were excluded (8 patients received multiple transplants, 9 patients were treated under pediatric protocols, 6 patients lacked research consent). 312/381 received voriconazole; other antifungal therapy included fluconazole (n=40), posaconazole (n=23), anidulafungin (n=1), and caspofungin (n=5). Median duration of cumulative days of voriconazole was 214 (range 2 -1553). SCC developed in 26/312 exposed to voriconazole and in 1/69 who received alternative antifungals. Cumulative incidence of SCC at 1 year was 3%, 2 years was 8%, 3 years was 13%, 4 years was 14%, and at 5 years was 19% (figure 1). Cumulative days of voriconazole use was found to be a risk factor for the development of SCC, and this relationship persisted in a multivariate model using previously identified risk factors (gender, age at transplant, TBI conditioning regimen, skin cancer pre-HSCT, chronic GVHD) as covariates (HR 1.859 for each 180 days of use, p<0.001). History of prior voriconazole exposure was not associated with an increased risk of SCC after covariate adjustment (HR 2.436, p=0.2369).
This is the first study to establish cumulative days of voriconazole use as a risk factor for SCC development following allo-HSCT, and may help guide appropriate antifungal prophylaxis in this patient population which is already at an increased risk of developing skin cancers.
No relevant conflicts of interest to declare.
Baseline Characteristics of 381 patients undergoing allogeneic HSCT
| Characteristic . | HSCT Patients (N = 381) . |
|---|---|
| Age (years) | |
| Median | 53 |
| Range | 19-71 |
| Gender | |
| Male | 222 (58.3%) |
| Race | |
| Caucasian | 358 (94.0%) |
| Primary malignancy | |
| Acute Lymphoblastic Leukemia | 50 (13.1%) |
| Acute Myeloid Leukemia | 137 (36.0%) |
| Myelodysplastic/ Myeloproliferative Disorders | 71 (18.6%) |
| Chronic Lymphoblastic Leukemia | 44 (11.5%) |
| Chronic Myelogenous Leukemia | 23 (6.0%) |
| Lymphoma | 17 (4.5%) |
| Plasma Cell Disorders | 30 (7.9%) |
| Non-Malignant Disorders | 9 (2.4%) |
| Fitzpatrick skin type | |
| 1-3 | 219 (57.5%) |
| 4-6 | 10 (2.6%) |
| Unknown | 152 (39.9%) |
| Skin cancer pre-transplant | |
| Melanoma | 8 (2.1 %) |
| Non-Melanoma | 25 (6.6 %) |
| Unknown/unavailable | 348 (91.3%) |
| Donor source | |
| HLA-matched related | 194 (50.9%) |
| HLA-matched unrelated | 141 (37.0%) |
| HLA-mismatched related | 2 (0.5%) |
| HLA-mismatched unrelated | 44 (11.5%) |
| Graft type | |
| Peripheral Blood | 331 (86.9%) |
| Bone Marrow | 40 (10.5%) |
| Umbilical Cord Blood | 10 (2.6%) |
| Conditioning Regimen | |
| Melphalan/Fludarabine | 130 (34.1%) |
| Cyclophosphamide/TBI | 106 (27.8%) |
| Fludarabine/TBI | 43 (11.3%) |
| Busulfan/Cyclophosphamide | 38 (10.0%) |
| Melphalan/TBI | 24 (6.3%) |
| Busulfan/Fludarabine | 11 (2.9%) |
| Cyclophosphamide/Fludarabine/TBI | 8 (2.1%) |
| Photophoresis/Pentostatin/TBI | 4 (1.0%) |
| Cyclophosphamide/Anti-Thymocyte Globulin | 3 (0.8%) |
| Busulfan/Fludarabine/Anti-Thymocyte Globulin | 3 (0.8%) |
| Other | 11 (2.9%) |
| GVHD prophylaxis | |
| Cyclosporine/Methotrexate | 159 (41.7%) |
| Tacrolimus/Methotrexate | 141 (37.0%) |
| Cyclosporine/Mycophenolate Mofetil | 27 (7.1%) |
| Tacrolimus/Mycophenolate Mofetil | 25 (6.6%) |
| Others | 29 (7.5%) |
| Total body irradiation | |
| Myeloablative | 137 (36.0%) |
| Reduced Intensity/Nonmyeloablative | 55 (14.4%) |
| Characteristic . | HSCT Patients (N = 381) . |
|---|---|
| Age (years) | |
| Median | 53 |
| Range | 19-71 |
| Gender | |
| Male | 222 (58.3%) |
| Race | |
| Caucasian | 358 (94.0%) |
| Primary malignancy | |
| Acute Lymphoblastic Leukemia | 50 (13.1%) |
| Acute Myeloid Leukemia | 137 (36.0%) |
| Myelodysplastic/ Myeloproliferative Disorders | 71 (18.6%) |
| Chronic Lymphoblastic Leukemia | 44 (11.5%) |
| Chronic Myelogenous Leukemia | 23 (6.0%) |
| Lymphoma | 17 (4.5%) |
| Plasma Cell Disorders | 30 (7.9%) |
| Non-Malignant Disorders | 9 (2.4%) |
| Fitzpatrick skin type | |
| 1-3 | 219 (57.5%) |
| 4-6 | 10 (2.6%) |
| Unknown | 152 (39.9%) |
| Skin cancer pre-transplant | |
| Melanoma | 8 (2.1 %) |
| Non-Melanoma | 25 (6.6 %) |
| Unknown/unavailable | 348 (91.3%) |
| Donor source | |
| HLA-matched related | 194 (50.9%) |
| HLA-matched unrelated | 141 (37.0%) |
| HLA-mismatched related | 2 (0.5%) |
| HLA-mismatched unrelated | 44 (11.5%) |
| Graft type | |
| Peripheral Blood | 331 (86.9%) |
| Bone Marrow | 40 (10.5%) |
| Umbilical Cord Blood | 10 (2.6%) |
| Conditioning Regimen | |
| Melphalan/Fludarabine | 130 (34.1%) |
| Cyclophosphamide/TBI | 106 (27.8%) |
| Fludarabine/TBI | 43 (11.3%) |
| Busulfan/Cyclophosphamide | 38 (10.0%) |
| Melphalan/TBI | 24 (6.3%) |
| Busulfan/Fludarabine | 11 (2.9%) |
| Cyclophosphamide/Fludarabine/TBI | 8 (2.1%) |
| Photophoresis/Pentostatin/TBI | 4 (1.0%) |
| Cyclophosphamide/Anti-Thymocyte Globulin | 3 (0.8%) |
| Busulfan/Fludarabine/Anti-Thymocyte Globulin | 3 (0.8%) |
| Other | 11 (2.9%) |
| GVHD prophylaxis | |
| Cyclosporine/Methotrexate | 159 (41.7%) |
| Tacrolimus/Methotrexate | 141 (37.0%) |
| Cyclosporine/Mycophenolate Mofetil | 27 (7.1%) |
| Tacrolimus/Mycophenolate Mofetil | 25 (6.6%) |
| Others | 29 (7.5%) |
| Total body irradiation | |
| Myeloablative | 137 (36.0%) |
| Reduced Intensity/Nonmyeloablative | 55 (14.4%) |
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal