Abstract
Hematopoietic stem/progenitor cells (HSPCs) residing in BM are released from their niches and circulate under steady-state conditions at detectable levels in the peripheral blood (PB), and their number increases in response to i) systemic or local inflammation, ii) strenuous exercise, iii) stress, iv) tissue/organ injury, and v) pharmacological agents. All these processes involve activation of the complement cascade (ComC), and while mice deficient in the complement protein C3, which is an upstream component of the ComC, are easy mobilizers (Blood 2004; 103:2071), mice deficient in a downstream component of ComC, complement protein 5 (C5), are very poor mobilizers (Leukemia 2009; 23:2052). To explain these observations, it has been suggested that during the mobilization process the C5a cleavage fragment stimulates release of proteolytic enzymes from BM-residing myeloid cells, which attenuates SDF-1–CXCR4 and VLA4–VCAM-1 retention signals in BM niches. In addition, C5a generated in BM sinusoids is a potent chemoattractant for granulocytes and monocytes, which, as the first cells egressing from the BM, play an important role in permeabilization of the BM–PB barrier and thus facilitate the subsequent egress of HSPCs. It is also known that activation of ComC is based on stepwise activation of the cascade of proteolytic pro-enzymes, and thus the lack of upstream C3 should theoretically affect generation of C5 convertase, which is a proteolytic enzyme activating a downstream component of ComC (C5). However, surprisingly, C3–/– mice are easy mobilizers (Blood 2004; 103:2071).
To explain how C5 can be activated during the mobilization process even when C3 is missing, we hypothesized that other proteases that are products of the activated coagulation cascade (CoaC) and fibrynolytic cascade (FibC) compensate for the lack of proteolytic activity of ComC-derived C5 convertase.
In our experiments 2-month-old C3-deficient mice (C3–/–) and normal wild type (WT) littermates were mobilized for 6 days by G-CSF in the presence or absence of selected CoaC and FibC inhibitors such as refludan (a direct inhibitor of thrombin) and tranexamic acid (an inhibitor of plasminogen activation). Following mobilization, we measured in PB i) the total number of white blood cells (WBC), ii) the number of circulating clonogenic CFU-GM, and iii) the number of Sca-1+c-kit+lineage– (SKL) cells. In parallel, we measured the activation of C5 by measuring the level of C5a and evaluated activation of CoaC by measuring prothrombin (PT) and activated partial thromboplastin times (APTT) as well as thrombin/antithrombin (TAT) and plasmin/antiplasmin (PAP) complexes.
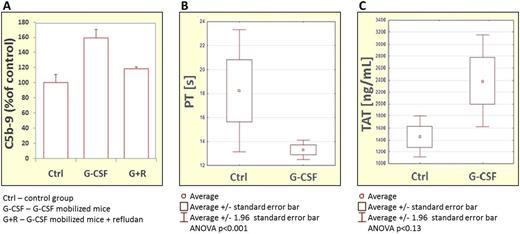
We observed that G-CSF-induced mobilization of HSPCs was significantly reduced in easy-mobilizing C3–/– mice if the mice were treated during mobilization with refludan (a CoaC inhibitor) or tranexamic acid (an FibC inhibitor). This reduction correlated with significant inhibition of C5 activation/cleavage. More importantly, we also noticed that inhibitors of CoaC and FibC had a negative effect on mobilization of HSPCs in normal WT animals. The activation of ComC, CoaC, and FibC in mice mobilized with G-CSF was confirmed by an increase in C5a level (Figure 1A) and by measuring PT and APTT time (Figure 1B) as well as TAT and PAP complexes (Figure 1C).
The data presented in this work demonstrate, for the first time, the existence of crosstalk between all three evolutionarily ancient proteolytic enzyme cascades, ComC, CoaC, and FibC, in the mobilization process of HSPCs. These results also confirm that C5, which plays an important role in egress of HSPCs from the BM, can be activated/cleaved during mobilization not only by ComC-generated C5 convertase but in addition by proteolytic enzymes of CoaC and FibC. Our observations of crosstalk between ComC, CoaC, and FibC may lead to the development of more efficient mobilization strategies in poor mobilizers. Furthermore, since it is known that all these cascades are activated in all the situations in which HSPCs are mobilized from BM into PB (e.g., infections, tissue/organ damage, or strenuous exercise) and show a circadian rhythm of activation due to a drop in blood pH during deep sleep at night, they are involved both in stress-induced as well as in circadian changes in HSPC trafficking in PB.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal