Abstract
Compared with growth factor (G) alone, the combination of G with the CXCR4 inhibitor plerixafor (G+P) increases peripheral blood CD34+ count and improves s CD34+ collection in multiple myeloma (MM) and lymphoma (Ly) patients undergoing autologous hematopoietic stem cell (AHSC) mobilization. It is unknown whether the improved CD34+ collection with G+P is attributed entirely to expansion of the circulating CD34+ pool or also to increased intra-apheresis CD34+ recruitment (replenishment of the circulating pool with cells from bone marrow) and consequently better collection efficiency (CE).
We retrospectively studied 192 patients with MM (N= 128) or Ly (N= 64), who underwent AHSC mobilization and collection with G (either filgrastim or pegfilgrastim, N= 73) or G+P (N=119) following a previously validated decision algorithm. Patients had undergone at least one session of leukapheresis of variable duration (typically 5 hours, independent of blood volume processed). Only data from first leukapheresis session was utilized in the analysis. CE, the average proportion of the circulating CD34+ pool that is captured for each blood volume processed, was calculated as CE = ( pCD34+)/( cCD34+ * nBV) , where pCD34+ is the number of CD34+ cells in the leukapheresis product, cCD34+ is the calculated circulating pool of CD34+ cells at beginning of apheresis and nBV is the number of blood volumes processed during leukapheresis. We investigated by multivariate analysis if any clinical factor, including use of plerixafor, influenced CE. To offset any possible effect of pre-apheresis CD34+ count in the peripheral blood (PB-CD34+) on CE, we compared CE between two sub-cohorts of G (N=59) and G+P (N=59) matched at 1:1 ratio according to pre apheresis PB-CD34+ count. We subsequently investigated whether there is a decline in CE with increasing number of blood volumes processed in either G or G+P.
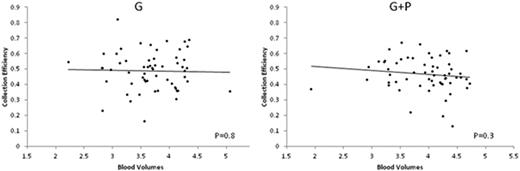
We found that neither age, gender, underlying disease (MM or Ly), type of growth factor utilized (filgrastim or pegfilgrastim), PB-D34+ count, number of blood volumes processed or use of plerixafor was significantly associated with CE. In the matched pair analysis, median CE was 0.50 (IQR 0.42-0.56) in the G group and 0.46 (IQR 0.41-0.54) in the G+P group (P=0.84), indicating that CD34+ recruitment during apheresis is similar in G and G+P. In both groups there was no significant change in CE across the range of nBV processed (Figure), suggesting that in both circumstances large volume leukapheresis can be performed without compromising collection efficiency. We then hypothesized that since all the impact of plerixafor on CD34+ in apheresis product can be reflected by PB-CD34+ with no distinct effect on CE, the use of plerixafor should not be predictive of the CD34+ collection when PB-CD34+ is included in the model. In fact, PB-CD34+ was a strong predictor of CD34+ collection (R2 = 0.83, P< 0.001) with no independent contribution of plerixafor to the model.
There is no difference in CE between patients mobilized with G or G+P indicating similar dynamics of intra-aphaeresis recruitment with both mobilization approaches. The lack of significant decline in CE across a wide range of nBV processed provides further rationale for large volume leukapheresis regardless of mobilization regimen utilized. PB-CD34+ count best reflects the impact of plerixafor and remains the strongest predictor of CD34+ collection.
Costa:Sanofi Aventis: Consultancy, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal