Abstract
Adult hematopoietic stem cells (HSCs) primarily reside in the hypoxic bone marrow microenvironment, and preferentially utilize anaerobic glycolysis to obtain energy. Cited2 is a cytokine-inducible gene, which plays various roles during mouse development. Our previous studies showed that deletion of Cited2 in adult mouse results in loss of HSC quiescence, increased apoptosis, and impaired HSC reconstitution capacity (Blood 2012, 119:2789-2798). In this study, we conditionally deleted Cited2 in Cited2fl/fl;Mx1-Cre mice and investigated the role of Cited2 in the metabolic regulation of HSCs.
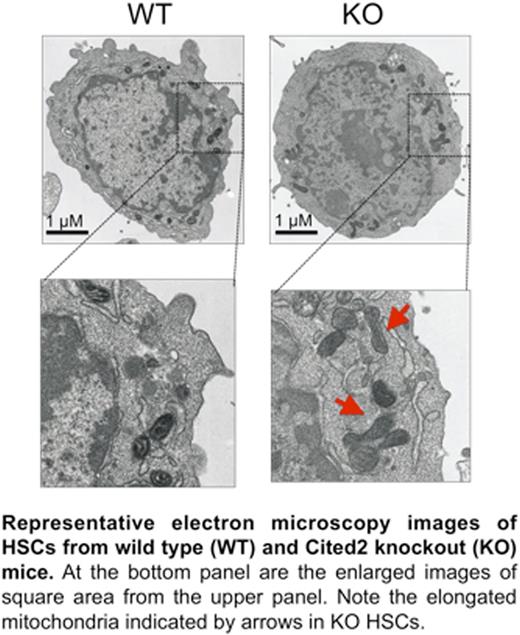
To further understand the metabolic changes in Cited2 KO HSCs, we measured glucose uptake using fluorescent indicator 2-NBDG. Glucose uptake was unchanged in the Cited2 KO LT- and ST- HSCs. Also, intracellular ATP content was maintained at the normal levels in Cited2 KO LT-HSCs, although slightly increased in ST-HSCs compared with WT controls. To assess the utilization of glycolysis in Cited2 KO HSCs, glycolytic flux was determined by glucose-derived 13C-lactate production using Gas Chromatography–Mass Spectrometry (GC-MS). We found that the rate of 13C-lactate production was significantly lower in both LT- and ST-HSCs lacking Cited2 than in WT controls. To further confirm this finding, we treated HSCs with antimycin A (AMA), a specific inhibitor of mitochondrial electron transport chain. We found that Cited2 KO HSCs displayed increased NADH after AMA treatment, compared with the WT control, indicating that mitochondrial respiration was increased in KO HSCs and produced more NADH.
At the molecular level, deletion of Cited2 significantly reduced the expression of metabolism related genes in HSCs, such as lactate dehydrogenase (LDH) B and LDHD, pyruvate dehydrogenase kinase (Pdk) 2 and Pdk4, PYGL (phosphorylase, glycogen, liver), and GPX1 (glutathione peroxidase 1). Notably, Pdk2 and Pdk4 were recently shown to be critical controllers of glycolysis and checkpoint for cell cycle in HSCs. Consistent with reduced expression of Pdk, the phosphorylation of PDH-E1α was significantly decreased in Cited2 KO HSCs.
Akt, mTOR, and FoxOs are known regulators of mitochondrial functions in HSCs. We found that Akt-mTOR signaling activity was increased in Cited2 KO HSCs, as indicated by increased phosphorylation of Akt and S6 ribosomal protein. However, in vitro treatment of LT-HSCs with mTORC1 inhibitor rapamycin did not resume decreased expression of LDHB, LDHD, Pdk2, and Pdk4, suggesting that elevated mTORC1 activity may not be the major contributor to the downregulation of glycolysis related genes. Meanwhile, we also found that in Cited2 KO LT-HSCs, phosphorylation of FoxO1 and FoxO3 was increased, both of which are known regulators of Pdk4 expression. Interestingly, in vitro treatment of LT-HSCs with PI3/Akt inhibitor LY294002, partially rescued the expression of Pdk4. Together, these findings suggest that the downregulation of Pdk4 in Cited2 KO HSCs is likely mediated by the inactivation of FoxOs caused by the elevated Akt activity.
In summary, these results show that loss of Cited2 attenuates HSCs' glycolytic metabolism while simultaneously enhancing their overall mitochondrial oxidative phosphorylation, thus suggesting a critical role of Cited2 in the maintenance of adult HSC glycolytic metabolism likely through regulating LDH, Pdk, and Akt activity.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal