Abstract
Treatment for vaso-occlusive crisis (VOC) in sickle cell disease (SCD) remains supportive, focusing largely on symptom relief with opioids. Animal models have suggested a role for both red cell and leukocyte selectin-mediated adhesion in VOC, and the pan-selectin inhibitor GMI 1070 restored blood flow in a mouse model of VOC. We therefore explored safety and possible efficacy of GMI 1070 in patients with VOC.
This prospective, randomized multi-center double blind, adaptive Phase 2 study enrolled patients ages 12-60 yrs with HbSS or HbSβ0 thalassemia presenting with VOC. Patients were randomized 1:1, stratified by site. A loading dose of GMI 1070 to achieve steady state, followed by ≤14 subsequent doses, were given q 12 h. Other treatment was per institutional standard of care. The primary efficacy endpoint was resolution of VOC, defined as: 1.5 cm sustained decrease in visual analog scale (VAS) pain score from baseline and transition to oral analgesia; or documented readiness for discharge; or order for discharge. Pre-planned population PK was examined after 11 subjects, and dose was subsequently escalated, per protocol. Secondary endpoints included cumulative opioid use (morphine equivalent units [MEU]/kg) and adverse events. Differences by treatment were analyzed by ANCOVA (means, adjusted for age and sex) and Kaplan-Meier (KM, medians), stratified by age and dose.
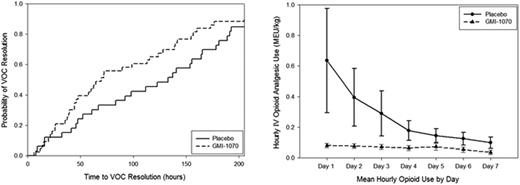
Seventy-six subjects were enrolled (ages 12-51; 45 F, 31 M). Drug dose was doubled to reach target nadir levels after interim PK analysis. All 76 patients reached the primary endpoint: 58 subjects continued study drug until criteria for resolution of VOC were met or the maximum number of doses were given; 18 discontinued drug for AEs, no improvement at day 5, or other reasons. In the primary efficacy analysis, reduction in mean time to VOC resolution was consistently observed in all treatment groups (pooled high and low dose GMI 1070, 103.6 h ± 20.9 (SE), n=43, vs placebo, 144.6 h ± 23.5, n=33, p=0.192). KM analysis showed median times to resolution of 69.6 h (CI 44.3, 115.5) for the GMI 1070 group vs 132.9 h (CI 67.0, 164.2) for the placebo group (p=0.187, Log-rank, Fig 1a). Thus, GMI 1070 resulted in differences in time to resolution of 41 h (mean) and 63 h (median). Similar differences were seen when analysis was stratified by age. Proportionally more subjects receiving GMI 1070 than placebo achieved resolution of VOC at 48, 72, 96 and 120 h. Analyses by how endpoint was reached were consistent with composite findings. The time to transition to oral pain medications was substantially decreased in all treatment groups: this reduction was 76 h in the GMI 1070 group (KM p=0.089) and reached statistical significance in the pediatric population (87.8 h, KM p=0.037). Time to discharge was reduced from a median of 156.1 h (CI 75.4, 185.8) for placebo to 72.2 h (CI 59.9, 121.0) for GMI 1070 (p=0.092). Stratified analysis by age and dose showed similar reductions in time to discharge. Although 24 subjects (31.6%) did not achieve a sustained reduction in VAS while hospitalized, active treatment was associated with a decrease in time to first sustained reduction in VAS. Median times to VAS reduction were: GMI 1070, 72.0 h (CI 34.5, 115.7); placebo, 125.3 h (CI 19.7, 180.2). Time to agreement about readiness for discharge was documented in 88% and 85% of the treatment and placebo groups, with medians of 72.5 h (CI 60.9, 139.1) and 137.4 h (CI 83.2, 165.7), respectively (p=0.151). An 83% reduction in mean cumulative IV opioid analgesic use (mg/kg MEU) was seen across all treatment groups (p=0.010, Fig 1b). The effect on opioid use was seen within 24 h. Total adverse event (AE) rates, serious AEs, and ‘treatment related’ AEs were comparable across all groups and ages (high dose, low dose, and pooled GMI 1070, or placebo; pediatric vs. adult). No subjects discontinued the study due to AEs.
Treatment with GMI 1070 was associated with substantial reductions in mean and median time to resolution of VOC, analyzed either as a composite or component endpoints, that were near statistical significance. The magnitude of reduction was clinically meaningful. GMI 1070 was also associated with reduction in both hourly and daily opioid use. There was no evidence for heterogeneity of effect among patient groups. These promising results support a role for selectins in the pathophysiology of VOC and justify a Phase 3 study of GMI 1070 for SCD patients with VOC.
Telen:Dilaforette, NA: Research Funding; Pfizer, Inc.: Consultancy; GlycoMimetics, Inc.: Research Funding. Wun:Emmaus, Inc.: Clinical Adjudication Committee Other; Pfizer, Inc.: Consultancy; GlycoMimetics: Research Funding. McCavit:GlycoMimetics, Inc.: Research Funding; Pfizer, Inc.: Consultancy. De Castro:GlycoMimetics, Inc.: Research Funding; Novella Clinical: Consultancy. Krishnamurti:GlycoMimetics, Inc.: Research Funding. Lanzkron:GlycoMimetics, Inc.: Research Funding. Hsu:GlycoMimetics, Inc.: Research Funding. Smith:GlycoMimetics, Inc.: Research Funding. Rhee:Rho, Inc.: Employment; GlycoMimetics, Inc.: Research Funding. Magnani:GlycoMimetics, Inc.: Employment, Equity Ownership. Thackray:GlycoMimetics, Inc.: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal