Abstract
Bu-based conditioning regimens are commonly used prior to autologous hematopoietic stem cell transplantation (ASCT) for lymphoma, but clinical results of an intravenous (IV) Bu-based regimen have been limited to single center studies. This multi-center, single-arm, Phase 2 study prospectively evaluated the safety and efficacy of the IV BuCyE regimen in lymphoma patients undergoing ASCT.
The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), transplant-related mortality (TRM) and overall response rate. TRM was defined as a death after transplant due to any cause other than disease progression. Subjects underwent first ASCT for Hodgkin lymphoma (HL) and B-cell non-Hodgkin lymphoma (NHL) that relapsed after initial therapy, was initially refractory to an anthracycline-based chemotherapy, or for high-risk NHL histology in first complete remission (CR) [International Prognostic Index (IPI) score of 4-5, or mantle cell lymphoma (MCL)]. Eligible subjects achieved CR or partial remission (PR) following salvage chemotherapy. The study initially enrolled subjects of 18-80 years, but the protocol was amended to reduce the upper age limit to 65 years due to a high TRM rate at post-transplant 100 days for the subjects >65 years. Therefore, we report safety for all the subjects undergoing ASCT (n=203) and efficacy from those 65 years old or younger (n=186). IV Bu doses were individually adjusted based on pre-conditioning test PK results; the area under the concentration-time curve of Bu was targeted to 20,000 mM*min. Bu was given as a 3-hour infusion once daily from Days -8 through Day -5. E (1.4 g/m2) was administered on Day -4, followed by 2.5 g/m2/day of Cy on Days -3 and -2.
A total of 207 subjects with HL (n=66) or NHL (n=141) were enrolled from 32 centers in the US and Canada between February 2010 and April 2012. Four subjects did not proceed with ASCT due to insurance or eligibility issues; the remaining 203 underwent ASCT. The final TRM rates at Day 100 for all subjects (n=203), for those older than 65 year old (n=17), and for those 65 years old or younger (n=186) were 4.5% (95% confidence intervals (CI) 2.1-8.3%), 23.5% (95% CI; 6.8-49.9%) and 2.7% (95% CI: 0.9-6.2%), respectively. The most common grade (Gr) 3 or 4 adverse events (CTCAE v3.0) observed from Day -8 through Day 100 were febrile neutropenia (Gr 3: 58.1%; Gr 4: 3.0%), stomatitis (Gr 3: 40.9%; no Gr 4), nausea (Gr 3: 8.9%; no Gr 4), and hypophosphatemia (Gr 3:6.9%; Gr 4: 1.0%). There was no instance of seizure or hepatic veno-occlusive disease (VOD) meeting the Baltimore criteria.
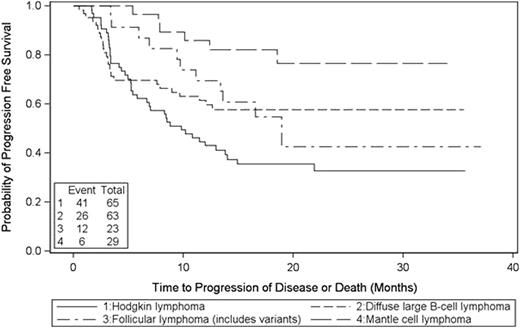
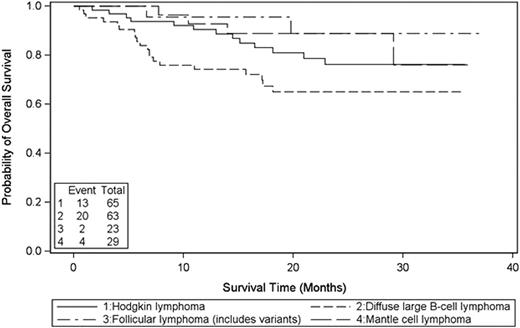
Efficacy was analyzed for 186 subjects ≤65 years old with HL (n=65) or NHL (n=121), which included diffuse large B-cell lymphoma (DLBCL; n=63), MCL (n=29) and follicular lymphoma (FL; n=23). The median age was 49 year old (range: 19-65); 36 % were female, 87% were white, 76% had a Karnofsky Score ≥90. Majority of the patients had CR2 or higher, or PR at transplant except that 19 MCL patients and three DLBCL patients with IPI score 4 had CR1. In addition, five patients (3 for NHL; 2 for HL) who had refractory disease to initial chemotherapy and required salvage therapy to achieve CR1 also enrolled this study. With median 20 months follow-up, the estimated 2-year PFS was 33% for HL and 58%, 77%, and 43% for DLBCL, MCL, and FL respectively [Fig. 1]. The estimated 2-year OS was 76% for HL and 65%, 89%, and 89% for DLBCL, MCL, and FL respectively [Fig. 2
].
IV BuCyE regimen provided good early disease control with acceptable safety profiles in B-cell NHL lymphoma patients 65 year old or younger, but there were early declines in PFS in HL patients. Additional comparisons of PFS and OS from the BuCyE regimen with a pre-specified, matched-control group of approximately 800 matched cases (1:4 ratio) obtained from the Center for International Blood and Marrow Transplant Research registry data who received carmustine, E, cytarabine, and melphalan (BEAM) conditioning regimen are underway, and updated results will be presented.
Off Label Use: Busulfan, Cyclophosphamide and VP-16 for Autologous Hematopoietic Stem Cell Transplantation. Costa:Otsuka: Research Funding. Freytes:Otsuka America Pharmaceutical: Research Funding, Travel funds for scientific presentation Other. Armstrong:Otsuka: Employment. Smith:Otsuka Pharmacetical Development & Commercialization: Employment. Elekes:Otsuka Pharmaceutical: Employment. Kato:Otsuka Pharmaceutical Development & Commercialization, Inc: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal