Abstract
Although prospective randomized studies have identified no demonstrable benefit for treatment of acute GVHD with methylprednisolone at doses >2 mg/kg/day, the minimum effective dose of methylprednisolone has not been defined. In a previous retrospective comparison of patients who initiated acute GVHD-treatment with prednisone-equivalent doses of 1 mg/kg/day or 2 mg/kg/day, the cumulative prednisone doses at day-42 of treatment were 31 mg/kg and 58 mg/kg, respectively (Mielcarek et al., Blood 2009). We therefore conducted a prospective phase III study to test the hypothesis that initial treatment of acute GVHD with “lower-dose” prednisone (prednisone-equivalent doses ≤1 mg/kg/day) is effective and safe.
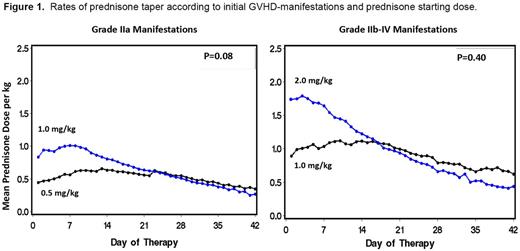
Patients with newly diagnosed acute GVHD (≥ Grade IIa) after allogeneic hematopoietic cell transplantation between 2009 and 2013 were eligible for the study. Patients were stratified according to severity of GVHD at symptom-onset. Patients with Grade IIa manifestations (upper gastrointestinal symptoms of anorexia, nausea, vomiting attributed to acute GVHD, with stool volumes <1 L/day, rash involving <50% of the body surface, and no hepatic dysfunction) were randomized to initiate GVHD-therapy with a prednisone-equivalent dose of either 1 or 0.5 mg/kg/day. Those with Grade IIb-IV manifestations (rash involving ≥50% of the body surface, stool volumes ≥1 L/day or hepatic involvement with total serum bilirubin >2 mg/dL) were randomized to start treatment with a prednisone-equivalent dose of either 2 or 1 mg/kg/day. Medications administered for GVHD-prophylaxis were continued as tolerated, and oral beclomethasone dipropionate and budesonide were typically used in combination with systemic glucocorticoids in patients with gastrointestinal GVHD. The rate of prednisone withdrawal was not prescribed by the protocol. The primary endpoint of the study was a ≥33% reduction of cumulative prednisone exposure by day-42 after initiation of treatment among patients given lower-dose prednisone compared to those given higher-dose prednisone. Measures of prednisone toxicity (infections, hyperglycemia) and possible harm (progression to Grade III/IV GVHD, secondary therapy for refractory GVHD, non-relapse mortality, recurrent malignancy) were secondary endpoints.
One-hundred and fifty patients were enrolled on the study (Grade IIa manifestations, n=92; Grade IIb-IV manifestations, n=58). For patients with Grade IIa GVHD treated initially with either 1 or 0.5 mg/kg, the cumulative prednisone doses at day-42 of treatment were 27.1 vs 22.2 mg/kg, respectively (18% reduction; p=0.08). For patients with Grade IIb-IV GVHD treated initially with either 2 or 1 mg/kg, cumulative prednisone doses at day-42 were 41.3 vs 38.4 mg/kg, respectively (7% reduction; p=0.4) (Figure 1). With a median follow-up of 27 (1-48) months, there were no significant differences in the risks of non-relapse mortality, recurrent malignancy and overall survival among patients started on higher-dose compared to those started on lower-dose initial therapy (15% vs 15%; 17% vs 11%; 76% vs 77%, respectively). Patients with Grade IIb-IV GVHD who started treatment with lower-dose prednisone were more likely to require secondary systemic immunosuppressive therapy than those who started treatment with higher-dose prednisone (41% vs 7%, p=0.001), and a trend suggested an increase in the risk of progression to Grade III-IV acute GVHD (19% vs 7%, p=0.2). The risks of infection and measures of glycemic control were not affected by initially assigned prednisone dose.
The primary endpoint of the study (≥33% reduction of cumulative prednisone exposure by day-42) was not reached due to the evolving practice of rapid prednisone withdrawal in responding patients treated with the higher dose. For patients presenting with Grade IIa GVHD manifestations, initiating treatment with lower-dose prednisone (0.5 mg/kg/day) was safe and effective. For patients presenting with Grade IIb-IV manifestations, however, initial treatment with lower-dose prednisone (1 mg/kg/day) was associated with an increased likelihood of requiring secondary immunosuppressive therapy without adversely affecting survival.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal