Abstract
Telomeres are sequences at the ends of each chromosome that confer genomic stability. The presence of dysfunctional telomeres is associated with increased genomic abnormalities and tumorigenesis. In CLL, telomere length has been described as an independent prognostic factor (e.g. Roos et al., Blood 2008) but these analyses have been performed on heterogeneous patient cohorts and have not yet been confirmed within clinical trials.
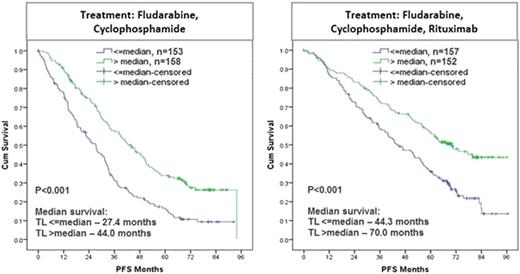
Here, we studied the impact of telomere length on outcome in the international CLL8 trial of the GCLLSG evaluating first-line treatment with fludarabine and cyclophosphamide (FC) versus FC with rituximab (Anti-CD20). Telomere length was analyzed using quantitative PCR of DNA (in triplicates) from baseline samples of 620 patients enrolled on CLL8, and this cohort was representative of the full trial population. The technique was validated using terminal restriction fragment length analysis (TRF) (R2=0.859, P<0.001) in an independent control sample set (n=18) and 6 of these samples were included in every batch as controls.
Telomere length was found to be highly variable in CLL (1.94 kb – 33.56 kb), whereas normal B-cells from age matched healthy probands (n=20) showed limited variability (5.39 – 9.60 kb; median 7.54 kb). Analysis of paired CD19+ (malignant) and CD19- (non-malignant) fractions (n=48) showed that telomere shortening in CLL was restricted to the malignant cell population (median: CD19+ 3.37 kb vs. CD19- 5.42 kb; P<0.001). Comparison of clinical characteristics between the groups defined by short and long telomeres (cut-off at the median of 4.54 kb) showed no significant associations with age (P=0.931), sex (P=0.116), presence of B-symptoms (P=0.297), ECOG status (P=0.288), CIRS score (P=0.438) and ß2-MG (P=0.586). Short telomeres were significantly associated with high WBC (≥50 Giga/L; P=0.004), high thymidine kinase levels (≥10.0 U/L; P<0.001) and presence of larger lymph nodes (>5cm; P=0.048). Also, unmutated IGHV (P<0.001), ZAP70+ (≥20%; P=0.005), del(17p) (P<0.001) and del(11q) (P<0.001) were significantly more frequent in the group with short telomeres. Interestingly, cases with Binet C stage were significantly associated with long telomeres (P=0.007).
In conclusion, the characterization of telomere length in the CLL8 trial revealed significant associations with other biological high-risk features and an independent prognostic impact on outcome after FC or FCR treatment. This points to a role of telomere dysfunction in CLL pathogenesis, progression and response to therapy. Further study of telomere dysfunction in the evolution of CLL and in the context of novel non-genotoxic treatments is warranted.
Wenger:F. Hoffmann-La Roche: Employment, Ownership interests (including stock options) in a start-up company, the stock of which is not publicly traded Other. Fingerle-Rowson:F. Hoffmann-La Roche : Employment. Wendtner:Hoffmann-La Roche: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Research Funding. Fischer:Roche: Travel grants Other; Mundipharma: Travel grants, Travel grants Other. Hallek:Roche: Consultancy, Honoraria, Research Funding. Stilgenbauer:Roche: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal