Abstract
Up-front autologous stem cell transplantation (ASCT-1) post front line therapy with novel agents is standard of care in newly diagnosed multiple myeloma. The role for salvage transplantation (ASCT-2) in relapsed patients after retreatment remains unclear in the era of the novel agents. Majority of published studies include patients treated in pre-thalidomide era. Our retrospective study investigates the safety and efficacy of ASCT-2 in patients exclusively treated with novel drugs both at upfront and at relapse. Primary end point was non relapse mortality (NRM) at day 100. Secondary end points were progression free survival from ASCT-2 (PFS-2) and overall survival (OS)
Thirty-nine patients (21 female and 18 male) underwent ASCT-2 at 4 centres between 2008 and 2013. At initial presentation all received thalidomide based treatments pre ASCT-1. Therapy at progression was bortezomib based in 92% and thalidomide in 8%. Melphalan 200 mg/m2 was used as conditioning for 90% of patients, 140 mg/m2 in 10%. OS and PFS-2 were calculated from ASCT-2. Statistical analysis was carried out using IBM SPSS 19 for Windows.
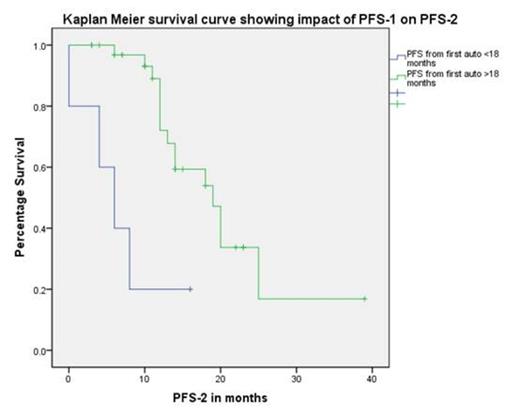
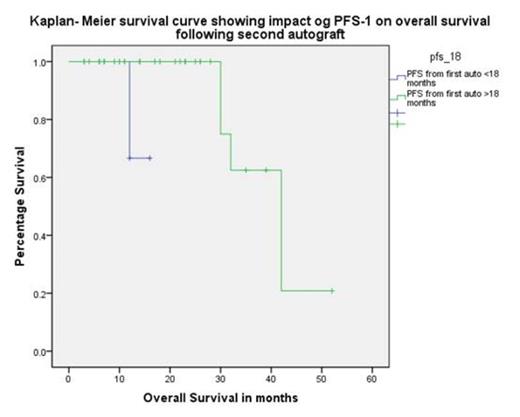
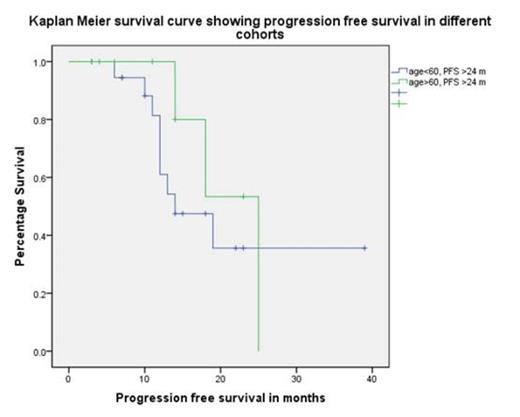
Median progression free survival (PFS-1) post ASCT-1 was 35 (10-90) months with 4 patients receiving thalidomide maintenance. Median age at ASCT-2 was 60 (37-68) years with a median stem cell dose of 2.7×106 (2-7) CD34 cells /kg body weight. All patients engrafted with median times to neutrophil (>0.5) and platelet (>20) engraftment of 12 days each with a day+100 and 1 year NRM of 0%. With a median follow up from ASCT-2 of 18 (3-52) months, the median PFS-2 was 18 (12-24) months and OS was 42 (33-50) months. PFS-1 of greater than 18 months was associated with prolonged PFS-2 (19 vs. 6 months, p=0.001, log rank), however there was no statistical difference observed for PFS-1 beyond 24 months. Similarly PFS-1 >18 months predicted for improved OS (39 vs. 14 months, p=0.007, log rank). Age at ASCT-2(>or <60yrs) had no impact on PFS-2 or OS. Patient age>60 and with PFS-1 of >24 months had a median PFS-2 of 25months as compared to 14 months in patient age <60 with a PFS-1 of >24 months.
In the era of novel agents ASCT-2 can be safely delivered with 0% 1 year NRM. PFS-1 greater than 18 months gives better PFS-2 and OS suggesting a definite role of this therapy in a selected population Age greater than 60 years does not have adverse impact on either PFS or OS. Thus ASCT-2 should be considered in treatment strategies at disease progression and warrants further prospective studies
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal