Abstract
Graft-vs-host disease (GVHD) remains a major complication of allogeneic hematopoietic cell transplantation (HCT). Acute GVHD results from activated donor T cells that infiltrate and damage target organs, producing an inflammatory state. In contrast, the pathophysiology of chronic graft-vs-host disease (cGVHD) remains poorly understood. cGVHD can follow acute GVHD or emerge de novo (>d+100). The clinical picture varies and manifestations can resemble autoimmune disorders. Because IL-17 has emerged as a principal cytokine involved in autoimmunity, Th17 cells have attracted much attention in the transplant community. While IFNg-producing Th1 cells appear to drive acute GVHD, the role of Th17 cells in the pathophysiology of GVHD has not been fully clarified.
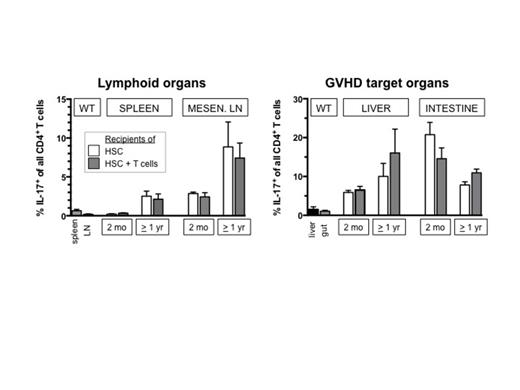
Here, we used an established minor-antigen disparate mouse model of acute and chronic GVHD to examine the emergence of IL-17+CD4+ Th17 cells post-HCT. Lethally irradiated BALB.B mice received pure hematopoietic stem cells (HSC; cKIT+Thy1.1loSca1+Lin-) or HSC plus splenic T cells from C57BL/6 donors (HSC: GFP; TC: CD45.1+). At several time points lymphoid and GVHD target organs were analyzed for donor T cell infiltration and T cell IL-17 expression. In this model recipients of HSC + T cells developed acute GVHD with intestinal involvement (diarrhea, weight loss) and a mortality of ∼30%, while mice given pure HSC remained healthy. Survivors stabilized around d45, but developed clinically evident chronic GVHD after 6-12 mo manifested by sclerodermatous skin excoriations and liver fibrosis/cirrhosis. Donor T cell infiltration of tissues (spleen, lymph nodes (LN), liver, intestines) was high at 2 and 4 wks post-HCT, but there was no detectable IL-17 production by CD4 cells during acute GVHD. The degree of donor T cell infiltration decreased (as acute GVHD improved) in these tissues. However, at 2 mo post-HCT higher percentages CD4+IL-17+ cells were observed, first in intestines and mesenteric LN, followed by liver and skin. At all time points post-HCT proportions of Th17 cells were higher in HCT recipients (of HSC +/- T cells) as compared to normal wild-type (WT) tissues. To summarize, our key findings are: (i) In our model acute GVHD was driven by adoptively transferred mature (CD4+) T cells that acquired a Th1 phenotype, whereas IL-17 producing donor cells were not detectable during this period. IFNg and T-bet are negative regulators of RORgT, the master regulator of Th17. Thus, this observation is consistent with the idea that in the presence of donor Th1 cells the development of Th17 cells is suppressed. (ii) The effect of Th1-related suppression of Th17 persisted beyond the acute phase: recipients of T-cell replete grafts that survived acute GVHD but later developed chronic GVHD did not demonstrate increased CD4+IL-17+ cells. In these mice, organ-infiltrating donor T cells were primarily adoptively transferred T cells, supporting the postulation that no plasticity exists between committed Th1 and Th17 cells. (iii) Signs of chronic GVHD were observed in animals that had not suffered from severe acute GVHD. In particular, in groups without acute GVHD we observed CD4+IL-17+ cells starting at 2 mo, peaking around 6 mo and which stabilized >1 yr post-HCT. In spleen and peripheral LN of these mice only low levels of CD4+IL-17+ cells were detectable, but their proportion was high in GVHD target organs (liver, intestines, skin). The susceptibility of organs appeared to change post-HCT with high proportions of CD4+IL-17+ cells in the intestines at 2 mo post-HCT that decreased over time. In contrast, CD4+IL-17+ cells in the liver increased later in the time course (Figures). (iv) A centrally important observation was that CD4+IL-17+ cells primarily originated from donor HSC, even in recipients of mature donor T cells. Likewise, recipients of pure HSC showed increasing proportions of Th17 cells over time, and could also manifest signs of cGVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal