Abstract
Among the standard tests for paraprotein estimation in myeloma (SPEP, immunofixation (SIFE), SFLC ratio, and 24-hour urine collection for UPEP and UIFE), 24-hour urine collection is the most cumbersome test. Previous studies evaluating its substitution with SFLC ratio and SPEP have significant limitations. Prior studies included variety of plasma cell dyscrasias, and patient heterogeneity prevailed with inclusion of both newly diagnosed and relapsed/refractory myeloma (MM). We have evaluated the question of whether SFLC ratio and SPEP can substitute for 24-hour urine collection in a more homogeneous population of advanced myeloma patients, where a shift in secretion from intact immunoglobulin to SFLC (free light chain escape) is observed.
We analyzed 116 patients with advanced MM that enrolled on one of several phase I clinical trials at our insititution between 08/2006-12/2012. The sensitivity of SFLC ratios, SPEP and SIFE were compared with UIFE. Subsequently, we assessed the linear relationship between SFLC dichotomized by abnormal ratio (<0.26 or >1.65) vs. 24-hr paraproteinuria.
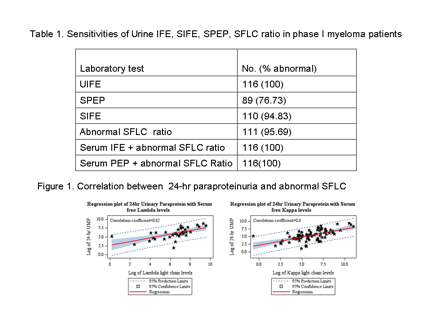
The sensitivities of SPEP, SIFE and abnormal SFLC ratio assays were 77%, 95% and 96%, respectively. The sensitivity of SFLC+SPEP/SFLC+SIFE increased to 100%, enabling detection of paraprotein in all patients (Table 1). In our second analysis, the quantification of SFLC correlated with 24-hr urinary paraprotein estimation. With every log increase in lambda and kappa light chains, 24-hr urinary paraprotein log increased by 0.52 times (p<0.0001) and 0.53 times (p<0.0001); correlation coefficients were 0.62 and 0.6, respectively (Figure 1).
In advanced myeloma patients, SFLC ratio in combination with SPEP or SIFE detected monoclonal paraprotein in 100% of patients. In addition, there was a linear correlation between SFLC and 24-hr urinary paraprotein estimation, suggesting there may not be a need for 24-hour collection. If these data are confirmed by other groups, we may be able to eliminate this cumbersome test in advanced myeloma patients.
Kaufman:Onyx: Consultancy; Celgene: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Janssen: Consultancy; Millenium: Consultancy; Merck: Research Funding. Gleason:Celgene: Consultancy. Heffner:Genentech: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Biotest: Honoraria, Research Funding; Onyx: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Pharmacyclics: Honoraria, Research Funding. Boise:Onyx: Consultancy. Lonial:Millennium: Consultancy; Celgene: Consultancy; Novartis: Consultancy; BMS: Consultancy; Sanofi: Consultancy; Onyx: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal