Abstract
IL-6 in the cancer microenvironment plays a crucial role in the constitutive activation of STAT3, which contributes to cancer cell progression. IL-6 binds to a heterodimer of membrane-bound receptors, IL-6R (CD126) and gp130, that triggers phosphorylation of JAK2 and downstream STAT3. The phosphorylated STAT3 acts as a transcription factor, binding target DNA and promoting tumorigenesis, cell proliferation and survival. IL-6 signaling pathways have been extensively studied as therapeutic targets in both cancer and autoimmune conditions. The anti-IL6R antibody Tocilizumab is a humanized anti CD126 antibody, approved by the FDA (Food and Drug Administration) for treatment of rheumatoid arthritis, that can block IL-6 classic and trans-signalling pathways. We investigated IL-6R expression in CLL cells and analysed the correlation of IL-6R expression with chemo-sensitization and the potential of targeting IL-6R to overcome drug resistance in vitro.
41 CLL patients were studied. Membrane-bound and soluble IL-6 receptors, CD126 and gp130, were measured by flow cytometry or ELISA. Constitutive activation of STAT3 and the expression of Bcl-2 family proteins were detected by western blotting. Cell cycle distribution was assessed with Ki67 and cell viability indicated by propidium iodide (PI) negative staining.
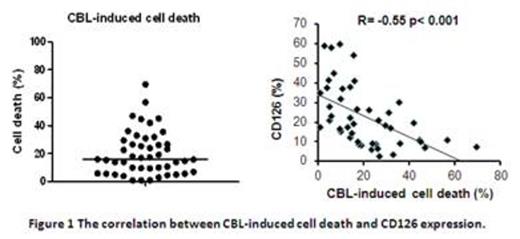
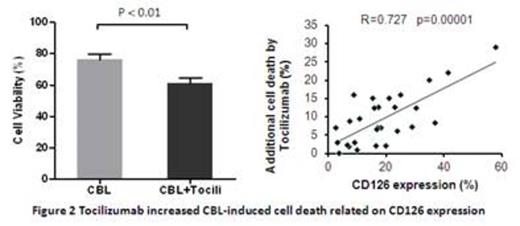
These results suggest that inhibiting IL6 signal pathways via IL-6R blockade with Tocilizumab is a new approach in CLL, leading to increased chemo-sensitisation to standard chemotherapeutic agents. Although CLL cells with higher CD126 expression exhibited more drug resistance in vitro, they also showed better response to Tocilizumab. Targeting the combination of Tocilizumab with anti-cancer therapy to CLL patients with high IL-6R expression might achieve clinical therapeutic efficacy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal