Abstract
Mutations in NOTCH1 (NOTCH1mut ) have been found in CLL with an incidence of about 10% and have been associated with unmutated IGHV, +12q, and poor outcome in previous studies. In the CLL8 trial (1st line FCR vs. FC), NOTCH1mut was identified as an independent unfavorable prognostic factor for progression free survival (PFS) and a predictive factor for reduced benefit from the addition of Rituximab to FC.
We assessed the incidence and impact of NOTCH1mut in the OMB110911 trial (1st line Chl vs. O-Chl) in patients considered inappropriate for fludarabine-based therapy. Pretreatment samples were available from 376 patients (84.1%) and this cohort was representative for the full trial population. The mutation hotspot fragment (chr9:139,390,619-139,390,840) of exon 34 of NOTCH1 was analyzed by Sanger sequencing and by NGS using Illumina MiSeq. NGS was used to evaluate the sensitivity of Sanger sequencing to detect c.7541_7542delCT mutation and to determine the exact variant frequency of the mutant allele.
The c.7541_7542delCT mutation was found by Sanger sequencing and by NGS in 45 (12.0%) of 376 patients (24 in O-Chl and 21 in Chl).
When comparing baseline characteristics, there were significant associations of NOTCH1mut with +12q (p=0.01), absence of 13q- (p=0.006) and unmutated IGHV (p=0.009), but not with gender, age , Binet stage, ECOG, CIRS, B symptoms, WBC, ß2-MG, 6q-, 11q-, and 17p-.
Regarding response to treatment, there was no association between NOTCH1mut and ORR or CR, neither in the whole group nor when analyzing the treatment arms separately.
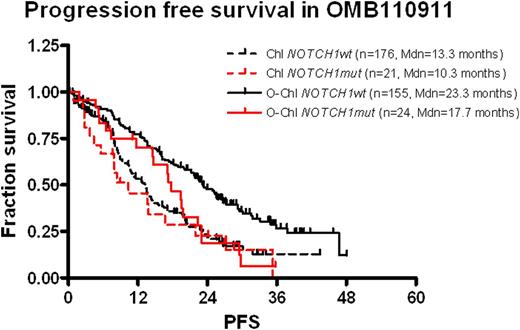
At a median follow-up of 29.0 months for PFS there were 249 events, at the medium follow-up of 31.7 months for OS 63 events in the 376 patient cohort. Similar to the full trial cohort, also in our cohort, O-Chl as compared to Chl resulted into significant improved PFS (median 22.4 vs. 13.1 months, HR=0.54, p<0.001). Of note, NOTCH1mut was associated with shorter PFS in the O-Chl arm (17.7 vs. 23.3, HR 1.86 p=0.01) but did not affect PFS in the Chl arm (10.3 vs. 13.3 months, HR 1.20 p=0.49). Correspondingly, in cases without NOTCH1mut a benefit from the addition of Ofatumumab was evident (HR 0.501 p<0.001) while for NOTCH1mut patients a reduced benefit which did not meet statistical significance was observed (HR 0.734 p=0.35).
To identify factors of independent clinical impact, we performed multivariable Cox regressions for PFS and OS including the following variables: treatment, gender, age, Binet stage, ECOG status, CIRS, B symptoms, WBC, ß2-MG, 11q, 17p, IGHV and NOTCH1. For PFS, the following independent prognostic factors were identified: O-Chl (HR 0.39, p<0.001), WBC > 50Gl/l (HR 2.66, p<0.001), CIRS Score >8 (HR 1.70 p<0.001), male gender (HR 1.39 p=0.04), unmutated IGHV (HR 1.38 p=0.04), 17p- (HR 3.19 p<0.001) and NOTCH1mut (HR 1.47 p=0.05).
Regarding OS, WBC > 50Gl/l (HR 2.58 p=0.01), ß2-MG > 5mg/l (HR 2.55 p=0.004), Binet Stage C (HR 2.13 p=0.01), 17p- (HR 4.97 p=0.001) and unmutated IGHV (HR 1.91 p=0.04) were identified as independent prognostic factors. Most likely due to the low frequency of NOTCH1mut of 12%, an interaction term in the multivariable model failed to achieve significance (HR 1.49, p=0.27).
When comparing NGS and Sanger sequencing, all cases with a mutant allele burden of >5% were detected by Sanger sequencing and in 34 of 45 NOTCH1mut patients, the hotspot mutation could be identified in a fraction >20%. For this subgroup, the effect of NOTCH1mut on PFS in the O-Chl treatment arm was even more pronounced (O-Chl: HR 2.459, p<0.01).
In the OMB110911 trial evaluating 1st line O-Chl against Chl, NOTCH1mut was associated with absence of 13q-, +12q, unmutated IGHV and a shorter PFS in multivariable analysis. Comparison of the impact of NOTCH1mut in both treatment arms suggests NOTCH1mut is a predictive marker for reduced benefit from the addition of Ofatumumab in the O-Chl treatment arm.
Tausch:GSK: Research Funding, Travel support Other. Off Label Use: First line Ofatumumab in combination with CBL in a clinical trial. Hillmen:GlaxoSmithKline: Honoraria, Research Funding. Offner:GlaxoSmithKline: Membership on an entity’s Board of Directors or advisory committees. Janssens:Mundipharma: Speakers Bureau; Roche: Speakers Bureau; GlaxoSmithKline: Speakers Bureau; Amgen: Speakers Bureau. Mayer:Roche: Consultancy, Research Funding; Glaxo: Consultancy, Research Funding. Panagiotidis:Roche: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; GSK: Consultancy, Honoraria. Danhauser-Riedl:GlaxoSmithKline GmbH & Co KG: Employment. McKeown:GSK: Employment. Winter:GlaxoSmithKline: Employment, Equity Ownership. Gupta:GSK: Employment. Stilgenbauer:GSK: Consultancy, Membership on an entity’s Board of Directors or advisory committees, Research Funding, Travel support Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal