Abstract
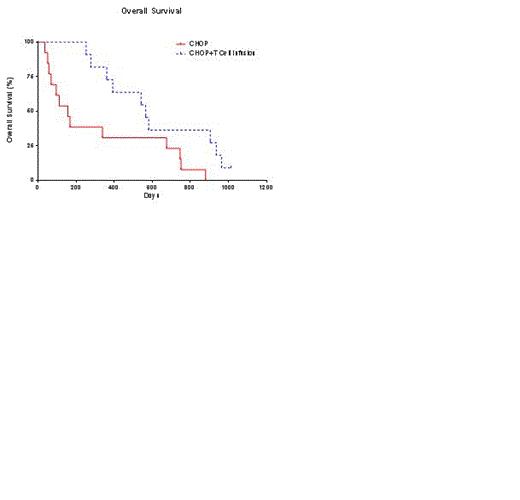
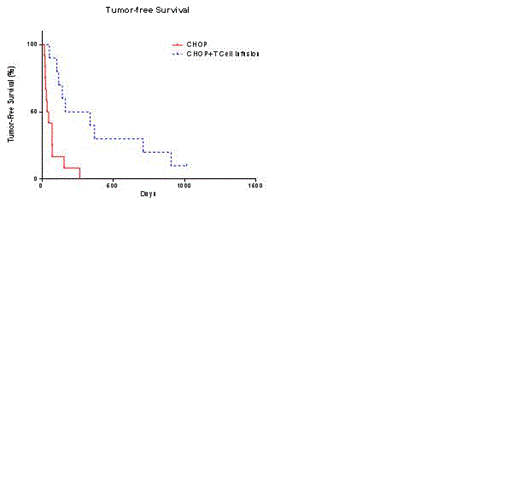
Companion canine cancer provides a clinically-relevant immunotherapy model for human malignancies because of their large size, intra-species genetic diversity, genetic similarity of tumors to human oncology, and spontaneously occurring tumors that develop despite an intact immune system. Canine B-lineage non-Hodgkin lymphoma (NHL) is genetically, molecularly, and physiologically similar to human NHL and the various treatment modalities can be interchanged with exception of antibody-based chemotherapies. Canine NHL represents 25% of all canine cancers diagnosed in the United States among the more than one million pet dogs diagnosed with cancer annually. As in humans, the common presentation is Stage III or Stage IV disease. It is rarely curable in the out-bred dog and even with aggressive chemotherapy the median survival rate is less than a year. Current standard-of-care (SOC) combination chemotherapy, CHOP, induces remission in approximately 85% of dogs. Thus, the companion canine provides a model for human T-cell therapy and addresses an unmet need of dogs diagnosed with NHL. Our initial canine trial (Sci Rep. 2012;2:249) demonstrated a survival advantage upon reconstitution of the immune system with autologous ex vivo-activated CD3+CD8+ T cells after CHOP for pet dogs with NHL. These T cells were propagated on engineered artificial antigen presenting cells (aAPC), which are also used for our human T-cell trials. Our updated survival data shows significant improvement in overall survival (p = 0.01) and tumor-free survival (p = 0.0008) compared with matched historical controls (Figure ). We also report initial data on an additional trial infusing donor- or patient-derived canine T cells after allogeneic or autologous hematopoietic stem-cell transplantation (HSCT), respectively. The first allogeneic and autologous recipients with NHL safely received 2 escalating doses of activated T cells and achieved an anti-tumor response. As in our first trial, granzyme B expression in the propagated T cells continues to significantly correlate with prognosis. A third trial tests whether steroid-resistant T cells can persist in pet dogs with NHL receiving systemic dosing of corticosteroids. Fluorescent dyes were used to distinguish infused T cells and demonstrated that steroid-resistant activated T cells survived compared to infused control T cells. In all trials, single-cell studies are underway to determine genetic factors concordant with the survival of infused T cells and treated companion canines. Global peripheral blood T-cell gene signatures, as quantitated by nanostring, were measured at 3 hours, 7 to 14 days, and 35 to 42 days post infusion. The different gene patterns between infused dogs and post infusion time points may reflect changes in en vivo T-cell activation and companion canine prognosis. In conclusion, the add-back of activated CD8+ T cells in dogs with NHL can inform of the immunobiology of adoptive transfer of T cells in humans.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal