Abstract
There is conflicting data on the effect of the addition of ATRA to chemotherapy in AML. Two large randomised trials showed no benefit (Estey et al Blood 1999; 93, 2478 (n=215); Burnett et al. Blood 2010, 115: 9482 (n=1075)), or benefit which was limited to patients with an NPM1 mutation (n=14) when given in combination with ICE (Idarubicin/Ara-C/Etoposide) (Schlenk et al, Leukemia 2004, 18; 1798 (n=242)) but not with DA alone. In an effort to prospectively clarify if this is predictive treatment for NPM1+ patients and whether the effect was etoposide dependent, we randomised 616 patients to DA vs ADE and ATRA vs no ATRA in a 2x2 factorial design.
Between August 2010 and May 2012, 616 patients were randomised. The median age was 67(53-82) years: 75% had de novo, 16% had secondary, and 8% had high risk MDS (marrow blasts 10-19%): 4%, 75% and 21% had favourable, intermediate or poor risk cytogenetics: ITD and NPM1 data was available on 422 and 404 patients, with mutation rates of 19% and 24%. A total of 56 patients (14% of those with data) were molecularly good risk (NPM1 mutant, ITD wild type). By Wheatley risk score, 24%, 40% and 36% had good, standard or poor risk disease. The demographic, cytogenetic, molecular and allocated treatments were balanced between the arms. Follow-up is complete to 1st January 2013 (median follow-up 18.7 months)
Patients were given Daunorubicin 50mg/m2 days 1-3 + Ara-C 100mg/m2 bid days 1-10 (course 1) or days 1-8 (course 2). Those allocated ATRA were treated at 45mg/m2/day for 60 days; Etoposide in the ADE arm was given at 100mg/m2/day on days 1-5 of courses 1 and 2 of chemotherapy.
The overall response rate (ORR) was 69% (CR 53%+ CRi 16%) and survival at 2 years was 35%. The ORR was not different between DA: 68% (CR 53%, CRi 15%) and ADE: 70% (CR 53%, CRi 17%), odds ratio (OR) 0.92 (0.65-1.30) p=0.6, although remission rates were non-significantly lower in patients given ATRA (ORR 66% (CR 53%, CRi 13%) vs 73% (CR 54%, CRi 19%), OR 1.39 (0.98-1.95) p=0.06) with significantly higher 30-day (16% vs 8%, p=0.005) and 60-day mortality (20% vs 12%, p=0.005). There were no differences in early mortality between ADE and DA arms. At 2 years, neither survival nor RFS differed between the arms: (ADE vs DA OS: 33% vs 36%, HR 1.07 (0.86-1.32) p=0.6; RFS: 23% vs 36% HR 1.15 (0.88-1.49) p=0.3; ATRA vs Not OS: 35% vs 35% HR 1.13 (0.91-1.40) p=0.3; RFS: 31% vs 30% HR 0.93 (0.71-1.20) p=0.6). Overall there was no interaction between the two treatments (OS, test for heterogeneity p=0.12).
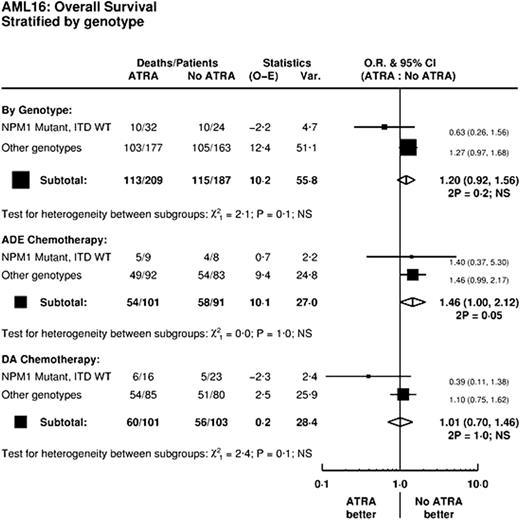
In an analysis stratified by Etoposide and by NPM1/ITD risk group there was no significant heterogeneity of the effect of ATRA (p=0.1, Figure). Importantly, when looked at by the underlying chemotherapy, no beneficial effect of ATRA in NPM1 mutant/ITD WT patients appeared for patients receiving ADE (p=1.0 for heterogeneity).
Neither the addition of Etoposide nor ATRA improves outcomes in this group of patients, with ATRA being associated with significantly greater early mortality. Importantly, an analysis by NPM1/FLT3 genotype fails to reproduce the large benefits seen by Schlenk et al in ATRA treated patients with an NPM1 mutant/ITD wild type genotype, either overall or for patients treated with ADE, thus failing to substantiate a benefit for ATRA in either context. Based upon the results of AML16, neither Etoposide nor ATRA improve outcomes for older patients given intensive DA chemotherapy.
This study received research support from Cancer Research UK, and the Cardiff Experimental Cardiff Medicine Centre.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal