Age specific differences in the function and concentration of haemostatic proteins are well established and are thought to contribute to the lower incidence of thromboembolism in infants and children compared to adults. We hypothesized that there are age-specific differences in the fibrin clot nanostructure and that these differences impact on fibrinolytic and anticoagulant treatment.
To determine the differences in fibrin clot nanostructure and response to tissue Plasminogen Activator (tPA) and Unfractionated Heparin (UFH) in infants compared to adults.
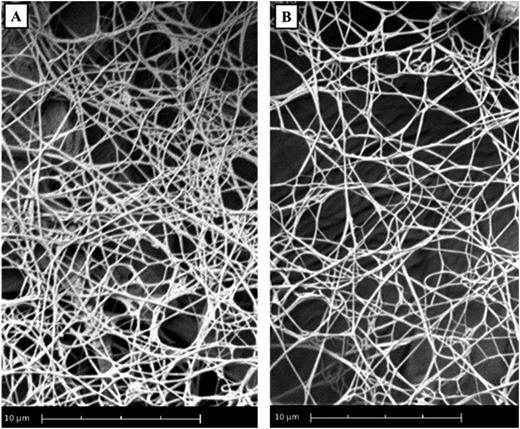
Four plasma samples from infants (0.86±0.16 years) and four samples from adults (32.04±15.80 years) were investigated. Thrombin generation (TG) was performed on paper discs in a microplate with 300mmol/L rhodamine substrate (P2Rho), 4mmol/L phospholipids, 5x10-9 mmol/L tissue factor and 16.7 mmol/L CaCl2; with and without additional 50ng/ml tPA, and 0.01IU/ml UFH. Fluorescence signal was recorded with a Fluoroskan Ascent fluorometer, with samples tested in triplicate. The resulting fibrin clots were fixed with 2.5% glutaraldehyde; washed with PBS and fixed with osmium tetroxide OsO4. The clots were dehydrated in ethanol and hexamethyldisilazane and were placed on carbon adhesive tabs. Each clot was coated with gold and studied using Scanning Electron Microscopy (SEM) on a Phenom Pro desktop SEM. The age-specific differences in fibrin fibre thickness and pore size were quantified using the Fibermetric software, with 36 representative images for infants and the same number for adults at each baseline, tPA and UFH, at magnification 10.000, counting 500 fibres per image.
We observed significant differences in fibrin clot nanostructure between infants and adults (Fig 1). Results are expressed as median and range. Fibrin fibers were thicker in infants compared to adults (343.3nm, 61.2nm to 997.1nm vs 352.3nm, 26.1nm to 997.5nm, p<0.001). In addition, the pore size was larger in infants (0.057pm2, 0.0041pm2 to 19.97pm2 vs 0.052pm2, 0.001pm2 to 15.24pm2, p<0.001).
In the presence of tPA in the adult sample, the fibrin fibers were thicker (352.3nm, 26.1nm to 997.5nm vs 355.8nm, 70.0nm to 999.6nm, p<0.05) and pore sizes larger (0.052pm2, 0.001pm2 to 15.24pm2 vs 0.049pm2, 0.0041pm2 to 20.26pm2, p<0.001) compared to baseline. Strikingly, in children, tPA did not induce changes in the fibrin fiber thickness (343.3nm, 61.2nm to 997.1nm vs 343.0nm, 65.6nm to 998.8nm, p>0.05) and pore size (0.057pm2, 0.0041pm2 to 19.97pm2 vs 0.050pm2, 0.0041pm2 to 19.34pm2, p>0.05).
In the presence of UFH in adults fibrin fibers were thicker (352.3nm, 26.1nm to 997.5nm vs 339.0, 66.9nm to 996.4nm, p<0.001) and pore sizes larger (0.052pm2, 0.001pm2 to 15.24pm2 vs 0.0540pm2, 0.0041pm2 to 21.87pm2, p<0.001) compared to baseline. In infants, in the presence of UFH, fibrin fibers were thicker (343.3nm, 61.2nm to 997.1nm vs 369.5nm, 109.1nm to 998.4nm, p<0.001), and pore sizes larger (0.057pm2, 0.0041pm2 to 19.97pm2 vs 0.055pm2, 0.0041pm2 to 24.25pm2, p<0.05).
Our study suggests that the fibrin clots in infants are weaker compared to those of adults and is consistent with the lower TG in infants. This is further supported by published evidence that clots produced with low thrombin concentrations are composed of thick fibrin fibres, whereas those generated with high thrombin concentrations are composed of a tight network of thin fibres.
The effect of both tPA and UFH on fibrin clots nanostructure in adults is consistent with published literature and is investigated in children for the first time. The fact that tPA did not have an effect on the fibrin clots of infants is not surprising considering that the concentration of plasminogen is 39%, tPA is 40% and TAFI function is 60% of the adult value; while PAI function is 50% higher compared to adults. UFH had an effect of the fibrin clot nanostructure in both infants and adults, with the effect on pore size being more pronounced in adults.
While developmental haemostasis has been understood for many years, this is the first study to provide definitive proof that the end product of coagulation is a different clot structure in children. This further enhances our understanding of why thrombosis are less common in children, and has significant implications for treatment.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal