Splenectomy is recommended by ASH and other guidelines as the main 2nd line therapy for ITP in patients who fail to respond or relapse after initial treatment with steroids. However, because of the possible peri- and postoperative complications, irreversibility and unpredictable response of splenectomy, medical alternatives that lead to avoidance of splenectomy are of great interest. Despite the lack of evidence-based data, rituximab (RTX) is now commonly used to manage ITP. RTX has not been properly evaluated in randomized placebo-controlled trials to determine its role as a splenectomy-sparing agent in ITP.
This study aimed to assess the efficacy of RTX against placebo in steroid- unresponsive ITP patients.
In this multi center (n=14) international, double blind placebo-controlled study, patients were randomly allocated to receive 4 weekly infusions of 375mg/m2 RTX or placebo. Steroids were allowed throughout the study. Main inclusion criteria were: 1- unsplenectomized patients with primary ITP with platelet count (PC) <30 or 30-50 x109/L if a higher PC was required, 2- failure to achieve sustained response to 1-2 mg/kg prednisone/prednisolone given ≥ 2 weeks (w) or relapse during steroid-tapering or after discontinuation. Exclusion criteria included previous administration of 2nd line treatments for ITP. The Primary endpoint was the rate of treatment failure within 78 w; treatment failure was defined as splenectomy or meeting criteria for splenectomy after w 12 if splenectomy was not be performed because of contraindication or patient's refusal. Criteria for splenectomy were defined as either PC <20 x109/L or a need of dose increment > 7.5 mg/day of Prednisone/prednisolone to maintain PC ≥20 x109/L. Secondary endpoints were rate of complete response (CR) at 24 w (PC >100 x109/L without administration of any platelet elevating therapy except steroids within the last 2 w) and safety.
Analysis was performed on intention to treat basis. The Kaplan-Meier method and logrank test were used for efficacy outcomes; all statistical tests were 2-sided with a significance level of 5%.
The study was approved by National Ethics Committees. Written informed consent was obtained from all patients.
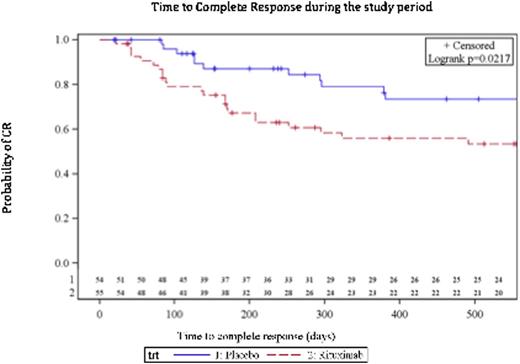
Characteristics, response and safety outcomes of the 109 randomized patients are shown in the table. Treatment with RTX did not reduce the rate of treatment failure at 78 w (logrank test p=0.6). However, there was a trend toward a reduction in the rate of splenectomy in the RTX-arm. Seventeen (31%) in the RTX-arm vs 6 (11%) in the placebo-arm achieved CR at 24 w (p=0.01). CR continued to occur after 24 w, figure. Although a number of patients in both arms relapsed during the follow-up, the difference in CR between the 2 arms remained significant throughout the study (p=0.02). RTX was associated with a higher rate of infections compared to placebo; one infection in each arm was evaluated as severe.
| . | Rituximab n= 55 . | Placebo n=54 . | . |
|---|---|---|---|
| Baseline characteristics | |||
| Age* (years) | 44 | 45 | |
| Female (n %) | 40 (73) | 39 (72) | |
| PC* x 109/L | 16 | 21 | |
| Treatment with steroids (n %) | 32 (86) | 24 (77) | |
| Duration of ITP* (days) | 262 | 354 | |
| Efficacy outcomes (n%) | p-value | ||
| Treatment failure at 78 w | 33 (58) | 32 (59) | 0.6 |
| Complete response at 24 w | 17 (31) | 6 (11) | 0.01 |
| Splenectomy | 10 (18) | 18 (33) | - |
| Safety outcomes | |||
| Death | 0 | 1 | - |
| Bleeding | 21 | 27 | - |
| Infections¤ | 22 | 13 | - |
| Thrombosis | 2 | 0 | - |
| . | Rituximab n= 55 . | Placebo n=54 . | . |
|---|---|---|---|
| Baseline characteristics | |||
| Age* (years) | 44 | 45 | |
| Female (n %) | 40 (73) | 39 (72) | |
| PC* x 109/L | 16 | 21 | |
| Treatment with steroids (n %) | 32 (86) | 24 (77) | |
| Duration of ITP* (days) | 262 | 354 | |
| Efficacy outcomes (n%) | p-value | ||
| Treatment failure at 78 w | 33 (58) | 32 (59) | 0.6 |
| Complete response at 24 w | 17 (31) | 6 (11) | 0.01 |
| Splenectomy | 10 (18) | 18 (33) | - |
| Safety outcomes | |||
| Death | 0 | 1 | - |
| Bleeding | 21 | 27 | - |
| Infections¤ | 22 | 13 | - |
| Thrombosis | 2 | 0 | - |
median
This is the first randomized placebo-controlled study aiming to assess both short and long-term efficacy and safety of RTX in steroid-unresponsive ITP patients. The study shows that treatment of steroid-unresponsive ITP with RTX did not reduce the rate of treatment failure, which was defind as a composite of splenectomy or meeting the predefined criteria for splenectomy if spenectomy was not performed. However, there was a trend toward a lower rate of splenectomy in the RTX-arm. Conversely, RTX resulted in a significnatly higher rate of CR at 24 w compared to Placebo - a difference that remained significant throughout the study.
In conclusion, monotherapy with RTX in steroid unresponsive patients did not alter the rate of treatment failure but yielded a significantly higher rates of CR as compared to placebo, which seems to be the main benift of treatment with RTX .
Ghanima:Roche: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; GSK: Honoraria. Off Label Use: Rituximab is not approved for the treatment of ITP. Michel:Roche: Honoraria, Research Funding; Amgen: Honoraria. Holme:Roche: Research Funding.
Author notes
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal