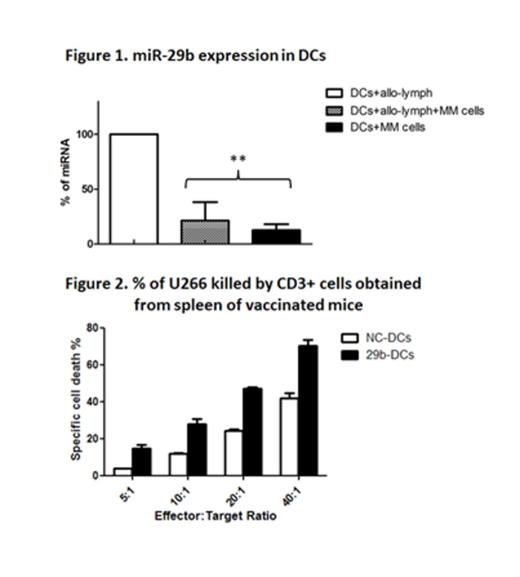
Dendritic cells (DCs) are potent antigen presenting cells that regulate the development of both innate and adaptive immune responses. According to their maturation status, DCs may ignite immune response or induce immune tolerance. Indeed, immature DCs (iDCs) present low levels of costimulatory molecules such as CD80 and CD86, and high levels of tolerogenic molecules such as B7H3. Upon exposure to maturation stimuli, DCs upregulate CD83 on their surface and gain the competence of stimulating T cell response. An efficient maturation is crucial for the generation of a specific cytotoxic T lymphocyte response, specially against cancer. However, recent reports have shown that Multiple Myeloma (MM) milieu can recruit DCs and reprogram them to sustain growth and survival of MM cells and protect them against immune response. Therapeutic approaches to restore DC functions rely on the identification of the pathways that are directly involved in induction of tolerance. Emerging evidence supports the role of microRNAs (miRNA) in the regulation of immune response and DC function. Among others, the miR-29 family seems to be involved in the modulation of NK activity, of Th1/Th2 phenotype switch and of DC differentiation from monocyte precursors. Besides, miR-29b targets and inhibits different and crucial immune-modulatory molecules such as B7H3, VEGF and IL-4. These findings suggest that miR-29 may play an important role in the multifaceted interplay between tumor cells and host’s immune system. To address this hypothesis, we generated iDCs from CD14+ monocytes of healthy donors and co-cultured them with: i) allogeneic (allo-) lymphocytes; ii) VEGF and IL-6 producing MM cells (RPMI8226 and U266); iii) allo-lymphocyte and MM cells. We found a consistent increase of miR-29b expression by RT-PCR during differentiation and maturation of DCs induced by allo-lymphocytes. However, when immature DCs were co-cultured with MM cells +/- allo-lymphocytes, a significant 3-fold reduction of miR-29b levels (p= 0.02) was observed (fig 1). This event occurred together with the absence of maturation markers, the persistence of high levels of B7H3 on the cell surface and with a raise in VEGF, IL-10, and IFN-gamma levels in the supernatant, confirming the MM-dependent impairment of the physiological DC maturation process. This latter concept was supported by the finding of increased number of CD4+CD25+Foxp3+T-regs in the DC/MM cell/allo-lymphocyte co-cultures as compared to the DC/allo-lymphocyte co-cultures (p= 0.05). To promote the recovery from the MM related immune-bias, we transiently transfected iDCs with miR-29b (29b-DCs) mimics or with a negative control (NC-DCs). We observed improved DC maturation (82.46% versus 39.89% of CD11c+/CD83+/CD86+ cells), reduced expression of B7H3 (33% reduction in MFI) and reduction of the T-reg number in 29b-DC/MM cell/allo-lymphocyte co-cultures as compared to NC-DC/MM cell/allo-lymphocyte co-culture. To investigate whether 29b-DCs were able to promote a specific CTL response against MM cells in vivo, we engrafted NOD/SCID γ chain-null mice with peripheral blood mononuclear cells (PBMCs) from HLA-A2+ healthy donors. DCs from the same donor were differentiated, transfected with either miR-29b or NC and then co-cultured with U266 for 48h. Mice were then vaccinated twice with either 29b-DCs or NC-DCs. Two weeks following the first injection, CD3+ human lymphocytes were recovered from mouse spleens (CD3 hu-splenocytes). We found an increased CD8/CD4 ratio in the CD3 hu-splenocytes collected from the 29b-DCs treated mouse as compared to control. To assess the capability of CD3+ hu-splenocytes to selectively kill U266 cells, we kept CD3 lymphocytes in culture in the presence of IL-15 for 48h. Then, we carried a cytotoxicity assay against U266 cell target. The highest specific lysis was attained with miR-29b DC primed CD3 hu-splenocytes (fig.2, p=0.03).
Taken together, our data indicate that: a) miR-29b regulates DC differentiation/maturation and function; b) MM cells reduce the expression of miR-29b in DCs, thus contributing to the establishment of an immune-permissive microenvironment; c) replacement of miR-29b within DCs partially restores their differentiation and functions in vitro and their capability to induce antitumor specific T-cell response in vivo. On these findings, miR-29b mimics are attractive candidates to enhance immunotherapy approaches against MM.
Disclosures:
No relevant conflicts of interest to declare.
© 2013 by The American Society of Hematology
2013

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal