The transcription factor aryl hydrocarbon receptor (AhR) is a cytosolic sensor of numerous small synthetic compounds (xenobiotics) and natural chemicals. Ligand binding of AhR causes a conformational change of the receptor, allowing its translocation to nucleus to regulate an array of genes. Different ligands could induce different sets of genes. AhR was first discovered as the mediator of dioxin toxicity. Its role in immunity was recently extensively investigated and expanded exponentially. Here we investigated the role of AhR in donor T cell-induced graft-versus-host diseases (GvHD) after bone marrow transplant in two MHC-mismatched models.
B6D2 or Balb/c recipients were irradiated with one dose (950 rads for B6D2 and 800 rads for Balb/c) one day before transplant. C57BL/6 WT or AhR deficient (AhR KO) T cells were transplanted with T cell-depleted bone marrow to B6D2 or Balb/c, at a dose of three million T cells or five hundred thousand T cells, respectively. At different time points, recipient lymphoid organs or target organs were harvested and used for flow analysis or ELISA. A group of recipient mice were observed for an extended time to establish GvHD scores and survival curves.
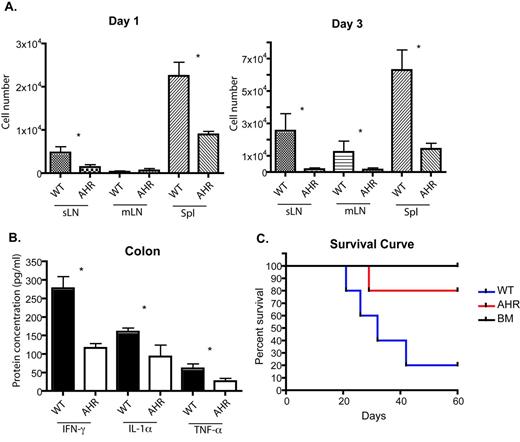
In B6 to B6D2 model, recipient mice transplanted with AhR KO T cells displayed lower GvHD scores and survived longer than mice transplanted with WT T cells (Figure 1C). We found that AhR KO donor T cells failed to accumulate in lymph nodes and spleen as rapidly as WT T cells as evident on day 1 and day 3 post-transplant (Figure 1A). Further investigation showed that the proliferation and cell death were similar between WT and AhR donor T cells, suggesting decreased migration of AhR KO T cells to lymphoid organs. It has been shown that 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induced AhR activation leads to the up-regulation of CCR4 and CCR5 in T cells, supporting our data that AhR affects cell migration. We also observed that lower percentage of AhR KO T cells produced IFN-g and IL-17 in lymph nodes and spleen early after transplant, but the differences disappeared at later time points (day 7). Colonic homogenates of AhR KO transplants contained less IFN-g, TNF-a, and IL-1a than WT transplants, which indicated that AhR KO T cells caused less inflammation in the gut (Figure 1B). Gut-associated injury due to inflammation is the major cause of death in this model.
A. CFSE-labeled donor B6 WT or AhR KO T cells were transplanted with T cell-depleted (TCD) BM cells to B6D2 recipients. 1 day and 3 days later, skin-draining LN (sLN), mesenteric LN (mLN), and spleens (Spl) were harvested and CFSE+ T cells were analyzed by flow cytometry. B-C. Donor WT or AhR KO T cells were transplanted with TCD BM cells. B. On day 7, B6D2 recipient mice were sacrificed and target organs were collected and homogenized. Cytokines were examined by ELISA. C. B6D2 recipient mice were observed over time for survival curve. The P-value between WT and AhR transplants survival curve was 0.0374 by Logrank test. * indicates statistically different by student t.
A. CFSE-labeled donor B6 WT or AhR KO T cells were transplanted with T cell-depleted (TCD) BM cells to B6D2 recipients. 1 day and 3 days later, skin-draining LN (sLN), mesenteric LN (mLN), and spleens (Spl) were harvested and CFSE+ T cells were analyzed by flow cytometry. B-C. Donor WT or AhR KO T cells were transplanted with TCD BM cells. B. On day 7, B6D2 recipient mice were sacrificed and target organs were collected and homogenized. Cytokines were examined by ELISA. C. B6D2 recipient mice were observed over time for survival curve. The P-value between WT and AhR transplants survival curve was 0.0374 by Logrank test. * indicates statistically different by student t.
However, in B6 to Balb/c model, recipient mice transplanted with AhR KO T cells displayed the opposite survival curve. They had diminished survival compared to mice receiving WT T cells with most mice succumbing to GvHD around day 10 to day 15 before the onset of diarrhea. AhR KO T cells in this model still accumulated less in lymph nodes at early time points, which suggested that AhR played a role in cell migration in both models. It was noteworthy that after day 6, the number of AhR KO T cells in the spleen was actually similar to or higher than WT T cells in both models, suggesting cell proliferation was not affected by AhR deficiency.
In conclusion, AhR KO T cells demonstrate decreased migration to lymphoid organs after bone marrow transplant compared to WT T cells. This decrease in migration may be the reason why mice transplanted with AhR KO T cells exhibit less inflammation in the gut and subsequently survive better from GvHD in the B6 to B6D2 model. However, mice transplanted with AhR KO T cells have diminished survival in B6 to Balb/c model despite of lower T cell numbers in lymph nodes at early time points. Thus, certain function of AhR in donor T cells is model dependent while other function is conserved among different models. These data suggest that investigators should be cautious regarding the supplementation of AhR ligands to diminish GvHD in clinical studies.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal