Abstract
Iron is a critical element for erythroid development and the function of many proteins, but is toxic in excessive amounts, most often by inducing oxidative stress that may play a role in vasculopathy. We have shown that markers of high iron burden such as serum ferritin have been associated with excessive production of the angiogenic factor placenta growth factor (PlGF), high estimated pulmonary artery pressure and death in adults with sickle cell disease. Enforced PlGF expression in mice promotes high circulating levels of the potent vasoconstrictor endothelin-1 and pulmonary hypertension.
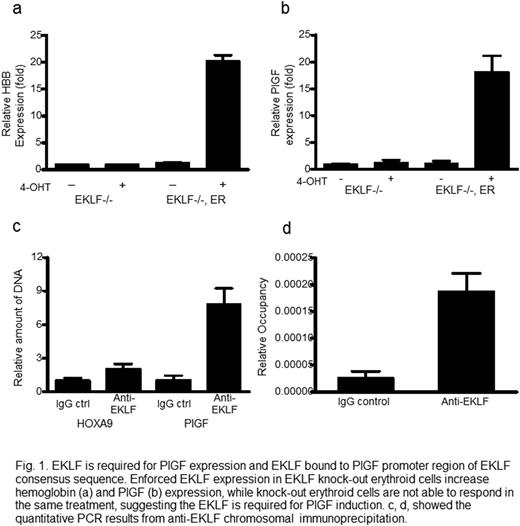
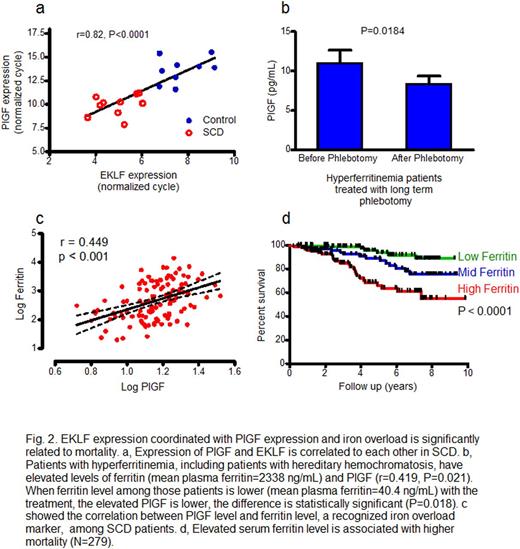
We find that heme-bound iron stimulates PlGF gene transcription by Erythroid Krüppel‐like Factor (EKLF). Heme-bound iron (hemin) induces PlGF mRNA in erythroid cells more than 200-fold in a dose-dependent and time-dependent fashion. PlGF mRNA is induced by other complexes of iron, but not iron-free heme or zinc protoporphyrin, suggesting that PlGF induction specifically requires iron. In murine and human cell lines, expression of EKLF developmentally precedes PlGF, and enforced expression of EKLF in human erythroid progenitor cells induces PlGF mRNA. Now we further report that hemin-induced expression of PlGF is abolished in EKLF-deficient mouse erythroid cells but rescued by conditional expression of EKLF. By chromosome immunoprecipitation we find that EKLF binds directly to the PlGF promoter region that contains an EKLF consensus sequence. We find that SCD patients have higher level expression than healthy controls of both EKLF and PlGF mRNA in peripheral blood cells, and expression of the two are correlated with each other (r=0.822, p<0.001).
Demonstrating the generalizability of this link of iron overload to PlGF expression, we find that patients with hereditary hemochromatosis have a high plasma level of PlGF, which falls after correction of iron overload by periodic phlebotomy therapy (p=0.018). Reductant N-acetyl cysteine completely blocks hemin induction of PlGF, supporting the participation of oxidant stress response pathways already known to mediate signaling by iron on other promoters such as heme oxygenase-1.
Our results for the first time demonstrate a specific mechanistic pathway induced by excess iron that is linked in humans with SCD and in mice to vasculopathy and pulmonary hypertension, suggesting that aggressive use of clinically approved iron chelation drugs should be investigated as adjunctive therapy to reduce the risk of pulmonary hypertension in SCD patients with associated iron overload. Linkage of iron overload in patients without hemolysis or anemia to abnormally high level expression of an angiogenic factor shown to induce pulmonary hypertension suggests a general pathobiological pathway connecting iron and vasculopathy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal