Abstract
The median age at diagnosis of MM is 69 years. Randomized, controlled studies on the safety and effectiveness of AHCT are lacking in those > 70 years of age and many patients are considered “ineligible” on the basis of age. We analyzed survival (OS) outcomes of 11,430 MM patients from US and Canada receiving AHCT after high dose melphalan (MEL) between 2008 -2011 reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). The relative efficacy of AHCT was compared in 3 cohorts; those aged ≥70 years (Cohort 1, n=946) vs. those 60-69 years (Cohort 2, n= 4666) and vs. 18-59 years (Cohort 3, n=5818). A statistically representative subset of 1279 patients was then analyzed in further detail to compare relapse, progression free survival (PFS) and non-relapse mortality (NRM).
The median ages in group 1, 2, and 3 were 72, 64 and 53 years, respectively with an upper age of 89 years. The older age cohort 1 was composed of a higher proportion of male patients, IgA MM, US patients (vs. Canada) and had worse Karnofsky scores (KPS < 100) and co morbidity scores (HCTCI ≥ 2), (all p values <0.05). The older age cohort was less likely to be transplanted within the first year of diagnosis and more likely to have MEL dose reduction (MEL <180 mg/m2 in 42%). Disease status at AHCT did not vary between groups with >40% of patients at least in a very good partial remission (≥VGPR) at transplant in all 3 cohorts.
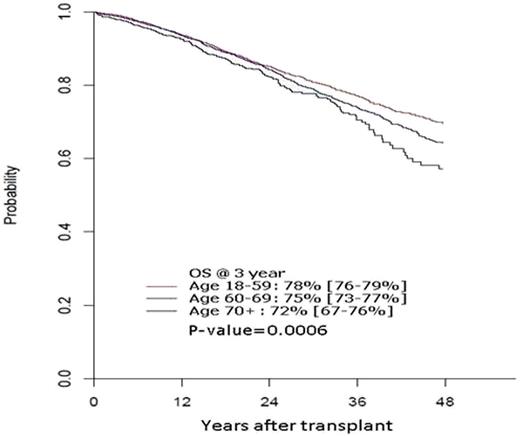
After a median follow up in survivors of 2 years, median OS has not been reached and 3 year OS was inferior for the older cohort at 72% (95% CI, 67-76%), 75% (73-77%), 78% (76-79%) in cohorts 2 and 3 respectively (Figure 1). In multivariate analysis, increasing age was associated with inferior OS (p=0.0006, Fig 1). Hazard ratio for death was 1.12 for cohort 2 vs. 3, 1.35 for cohort 1 vs. 3 and 1.2 for cohort 1 vs. 2. Other significant predictors of lower OS were higher HCTCI score, lower KPS, longer (>12mo) interval from diagnosis to AHCT and inferior disease status (<VGPR) at AHCT.
Further analyses were performed to identify the contribution of relapse, NRM and post relapse survival. NRM within the first year was 0% for the older cohort and 2% for the other 2 cohorts, likely reflecting careful patient selection. Relapse risk at 3 years was similar between cohorts 1,2 and 3 at 63% (48-74%), 51% (55-66%) and 56% (51-60%) respectively. PFS at 3 years was 33% (21-46%), 38% (33-43%) and 42% (37-46%) respectively. In multivariate analyses, increasing age was NOT associated with higher risk of relapse, NRM or lower PFS. KPS <80 was associated with higher risk of relapse, NRM and lower PFS. Other significant risks for relapse and lower PFS were longer interval from diagnosis to AHCT and a < VGPR disease state prior to AHCT. Post relapse survival was significantly worse for the older cohort (p=0.03, Figure 2). Post relapse survival was significantly worse with increasing age. For cohorts 1, 2 and 3 at 2 years, it was 54% vs. 50% and 63% and at 3 yrs 25% vs. 37% and 49% respectively (p=0.03, Fig 2).
AHCT although performed less frequently in the older MM patient, offers equivalent efficacy in and is associated with low NRM. Survival differences are driven partly by higher co-morbidities and lower post relapse survival. Myeloma related outcomes are similar when appropriate older patients are treated with aggressive therapy.
Gasparetto:Onyx: Membership on an entity’s Board of Directors or advisory committees; Millennium (2012): Membership on an entity’s Board of Directors or advisory committees, Speakers Bureau; Celgene ( 2012): Membership on an entity’s Board of Directors or advisory committees, Research Funding, Speakers Bureau. Lonial:Sanofi: Consultancy; BMS: Consultancy; Novartis: Consultancy; Celgene: Consultancy; Millennium: Consultancy; Onyx: Consultancy. Hari:Celgene: Consultancy; Onyx: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal