Abstract
In the primary analysis of the phase III COMFORT-I study, the JAK1/JAK2 inhibitor ruxolitinib (RUX) significantly improved splenomegaly, myelofibrosis (MF)-related symptoms, and quality of life (QoL) measures in patients (pts) with MF versus placebo (PBO). An additional analysis (median follow-up 51 weeks [wks)]) showed RUX was associated with a survival advantage versus PBO (Verstovsek et al. N Engl J Med2012). Longer term follow-up (median 102 wks) showed durable SV reductions and QoL improvements. At this time point, RUX continued to be associated with a survival advantage, despite all pts originally randomized to PBO having discontinued or crossed over to ruxolitinib shortly after the primary analysis (Verstovsek et al. ASH 2012. abstract 800). We report additional longer-term follow-up of pts from COMFORT-I.
In COMFORT-I, 309 pts were randomized (1:1) to RUX or PBO. RUX starting dose depended on baseline platelet count (100–200´109/L: 15 mg BID; >200´109/L: 20 mg BID). Doses could be titrated for lack of efficacy or excess toxicity as specified in the protocol. Pts receiving PBO could crossover to RUX after the primary analysis (ie, when all pts completed 24 wks and half the pts completed 36 wks of therapy) or at any time if they met protocol-specified criteria for worsening splenomegaly. The primary endpoint was the proportion of pts who achieved a ≥35% reduction in spleen volume (SV) at wk 24. Symptoms were assessed up to wk 24 using the modified MF Symptom Assessment Form v2.0. QoL continued to be evaluated beyond wk 24 using the EORTC QoL Questionnaire-Core 30 (QLQ-C30). Survival was estimated by Kaplan-Meier analysis according to original randomized treatment. To better understand the effect of crossover on the PBO arm, statistical modeling of survival was conducted using a generalized Gamma distribution.
Median follow-up was 149 wks at the time of this updated analysis. Of 155 pts originally randomized to RUX, 77 remain on therapy. Of 154 pts originally randomized to PBO, 111 crossed over to RUX; the median time to crossover was 41.1 wks (range, 5.1–65.4). At wk 24, pts originally randomized to RUX had a mean SV reduction of −31.6%; this was durable for pts who continued on RUX, where the mean SV reduction at wk 144 was −34.1%. Fifty-nine percent (91/155) of pts originally randomized to RUX achieved a ≥35% SV reduction at any time during study; for these pts, the probability of maintaining this response for >132 weeks from the time of their initial response was 0.53. Pts originally randomized to RUX who continued on therapy experienced durable improvements in the Global Health Status/QoL and functional domains of the EORTC QLQ-C30.
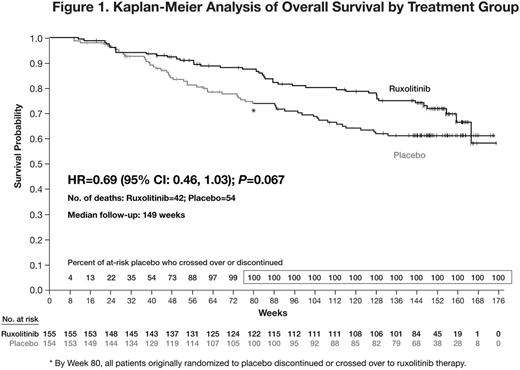
Overall survival favored RUX (HR=0.69; 95% CI: 0.46, 1.03; P= 0.067; Fig 1), with 42 and 54 deaths among pts originally randomized to RUX and PBO, respectively. Modeling of the hazard function of death over time to explore the effect of crossover to RUX in pts originally randomized to PBO showed a decrease in hazard for these pts, which corresponded to an increased proportion of pts crossing over to RUX.
In pts originally randomized to RUX who continued on therapy, there was no substantial change in the rate, distribution, or severity of nonhematologic adverse events (AEs) with longer-term therapy; most nonhematologic AEs were grade 1 or 2. Mean and median hemoglobin and platelet count remained stable with longer-term therapy. Consistent with this, the incidence of new onset grade 3 or 4 anemia and thrombocytopenia observed after the first 6 months of RUX therapy decreased relative to that seen in the first 6 months of therapy (Fig 2). With approximately 1 year of additional follow up, 4 new cases of acute myeloid leukemia were reported: 2 pts originally randomized to PBO (376 and 666 days after crossover to RUX) and 2 pts originally randomized to RUX (848 and 1143 days after starting RUX).
After a median follow-up of 149 wks, the hazard ratio for overall survival favored pts originally randomized to RUX over those originally randomized to PBO, the majority of whom crossed over to RUX. The hazard of death for pts originally randomized to PBO decreased as pts crossed over to RUX. SV reductions and QoL improvements were sustained with longer-term therapy. The incidence of new onset grade 3 or 4 anemia and thrombocytopenia decreased with longer-term therapy. Collectively, these data reinforce the durable efficacy and longer-term safety of RUX in pts with MF.
Verstovsek:Incyte Corporation: Research Funding. Mesa:Genentech: Research Funding; NS Pharma: Research Funding; Gilead: Research Funding; Incyte Corporation: Research Funding; Lilly: Research Funding. Gotlib:Incyte Corporation: Travel support, Travel support Other; Incyte Corporation: Membership on an entity’s Board of Directors or advisory committees; Incyte Corporation: Research Funding. Levy:Incyte Corporation: Equity Ownership; Incyte Corporation: Employment. Gupta:Incyte Corporation: Consultancy; Novartis: Consultancy; Inctye Corporation: Research Funding; Novartis: Research Funding; Novartis: Speakers Bureau. Deininger:BMS, ARIAD, NOVARTIS: Consultancy; BMS, NOVARTIS, CELGENE, GILEAD: Research Funding; ARIAD, NOVARTIS: Advisory Boards, Advisory Boards Other. Miller:Novartis: Consultancy; Incyte Corporation: Consultancy; Novartis: Research Funding; Incyte Corporation: Research Funding; Novartis: Honoraria; Incyte Corporation: Honoraria. Talpaz:Ariad: Consultancy; Novartis: Consultancy; BMS: Consultancy; Pfizer: Consultancy; Ariad: Membership on an entity’s Board of Directors or advisory committees; Novartis: Membership on an entity’s Board of Directors or advisory committees; BMS: Membership on an entity’s Board of Directors or advisory committees; Pfizer: Membership on an entity’s Board of Directors or advisory committees. Winton:Incyte Corporation: Research Funding; Incyte Corporation: Membership on an entity’s Board of Directors or advisory committees. Arcasoy:Incyte Corporation: Research Funding. Lyons:Lilly: Research Funding; Amgen: Research Funding; Celgene: Research Funding; Incyte Corporation: Research Funding; Gilead: Consultancy; Seattle Genetics: Research Funding; Gilead: Research Funding; Ariad: Research Funding; GlaxoSmithKline: Research Funding; Novartis: Research Funding. Paquette:Incyte Corporation: Consultancy. Raza:Novartis: Speakers Bureau; Onconova Inc: Research Funding. Vaddi:Incyte Corporation: Employment; Incyte Corporation: Equity Ownership. Sun:Incyte: Employment; Incyte: Equity Ownership. Peng:Incyte Corporation: Employment; Incyte Corporation: Equity Ownership. Sandor:Incyte: Employment; Incyte: Equity Ownership. Kantarjian:Ariad: Research Funding; Novartis: Research Funding; BMS: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal