Abstract
Core-binding factor (CBF) AML is associated with high response rate and favorable prognosis when treated with intensive chemotherapy (IC) including intermediate to high dose cytarabine (IHDAC). However, IC can be associated with high treatment-related mortality in elderly patients (pts). Combination of anthracycline and IHDAC in older (age ≥ 60) pts with CBF-AML can result in 88% complete remission (CR) rate but be associated with approximately 10% early treatment-related mortality (Prebet, Boissel et al. JCO, 2009). Thus, there is an urgent need to reduce treatment-related mortality while maintaining the response in this pt population. Our previous study showed that fludarabine and cytarabine based induction regimens, which do not include anthracycline, were highly effective in CBF-AML.(Borthakur, Kantarjian et al. Cancer, 2008), We investigated the response and treatment-related mortality of regimens based on this combination in elderly pts with CBF-AML.
Previously untreated CBF-AML pts (defined by the presence of t(8;21) or inv(16) ), ages ≥ 60, who received induction treatment under one of the clinical trials at MD Anderson (ID02-266 or 2007-0147) using fludarabine and cytarabine combination without conventional anthracycline were eligible for the analysis. Planned induction comprised of either FA (fludarabine 30 mg/m2 IV daily x 5 days followed by cytarabine 2g/m2 IV daily on the same days), FLAG (GCS-F 5µg/kg subcutaneously daily from day -1 to 5 in addition to FA), or FLAG+GO (gemtuzumab ozogamicin 3mg/m2 IV on day 1 in addition to FLAG). Consolidation therapy was given with the same regimen with reduced dose for total 6 cycles if tolerated.
In total, 34 pts (23 [68%] with inv(16) and 9[32%] with t(8;21)) were eligible for the analysis. Three (9%) pts received FA induction, whereas 18 (53%) and 13 (38%) received FLAG or FLAG+GO induction, respectively. Twenty six (76%) pts received full dose of induction regimen, whereas 8 (24%) pts received reduced dose of induction (from 20%-50% dose reduction). Thirty two (94%) pts were able to receive consolidation therapy for the median 5 cycles (range: 1-6). The median age of the cohort was 68 (range: 60-88). Seven (21%) pts had ECOG performance status (PS) ≥ 2. Twelve (35%) pts had other cytogenetic abnormalities in addition to CBF alteration. None of the pts tested had c-kit mutation (0/16). Other pt demographics are also described in Table 1.
CR and CR without platelet recovery (CRp) were documented in 30 (88%) and 3 (9%) pts, respectively (overall response rate [ORR] = 98%). Two of the 33 responders required 2 cycles to achieve the response. Early treatment related mortality at 4 weeks and 8 weeks was documented in 1 (3%) and 2 (6%) pts, respectively (both pts received FLAG). Ten out of 11 pts on FLAG+GO regimen with available follow up q-PCR data, achieved ≥3 log reduction in leukemia associated fusion transcript ratio by 3 cycles of therapy.
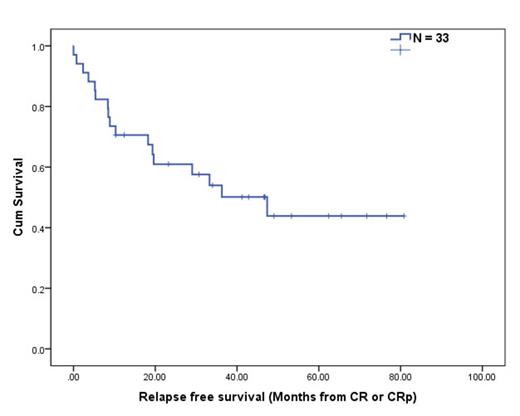
Six pts relapsed and one opted for stem cell transplant in remission. Median overall survival (OS) in the entire cohort was 47.9 months. The median relapse free survival (RFS: defined as time interval from CR/CRp to relapse or death) was 47.4 months (95% CI: 21.6-73.1, Figure 1), whereas 1 year and 2 year RFS rate was 73% and 63%, respectively. In an analysis subgrouped by the pt demographics, RFS was significantly worse in pts with PS ≥ 2 (vs. PS < 2, P = 0.03). Howerve, there was no statistical difference in RFS between 1) different age group (age < 70 vs. ≥ 70), 2) inv(16) vs. t(8;21), 3) additional cytogenetics, 4) induction regimen, and 5) presence of molecular alterations (FLT3-ITD, RAS, or NPM1 mutations).
Figure.
Induction therapy with fludarbine and cytarabine based combination regimen is highly effective (ORR 98%), provides durable remissions and is associated with low treatment-related mortality (3% in 4 weeks, 6% in 8 weeks) in elderly (age ≥ 60) pts with CBF-AML. Our findings suggest that anthracycline could be omitted from induction treatment in this pt population.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal