Abstract
Chemotherapy induced nausea and vomiting can be a significant problem for pts with acute myeloid leukemia (AML) receiving induction chemotherapy causing deterioration in quality of life. Cytarabine-containing regimens are the cornerstone for the management of AML and cytarabine is a moderate emetic risk chemotherapy agent. Combination of cytarabine with other chemotherapeutic agent can substantially worsen nausea and vomiting. Ondansetron has been used for nausea control in pts receiving chemotherapy. Aprepitant is a P/neurokinin-1 (NK1)-receptor antagonist approved for use in combination with a 5-HT3-receptor antagonist and dexamethasone for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. We explored whether the combination of two drugs with different mechanisms of action may improve the control of nausea in pts with AML receiving cytarabine-based induction.
Pts with AML, high-risk MDS or CML blast phase receiving induction chemotherapy with cytarabine at a dose of ≥1 g/m2/day for at least 3 days were randomized to: Arm 1) Ondansetron 8mg IV 30 minutes before high dose cytarabine followed by ondansetron 24mg IV continuous infusion daily until 6-12 hours after the end of last chemotherapy infusion. Arm 2) Aprepitant 125mg PO plus ondansetron 8mg IV 30 minutes before high dose cytarabine followed by ondansetron 24mg IV continuous infusion daily until 6-12 hours after the end of last chemotherapy, plus aprepitant 80 mg PO daily and to continue for 1 day after last dose of chemotherapy. Pts were followed for 72 hrs post chemotherapy. A diary was used by the pts to record daily number of episodes of nausea and /or vomiting experienced during the study, as well as to record any need of extra medications for escape nausea. Complete response was defined as no emesis episodes and no use of rescue medication during the study period. Partial response was defined as ≤ 1 episode of emesis during the entire study period, no use of rescue medication during the study period, and no more than moderate nausea (NCI grade 2).
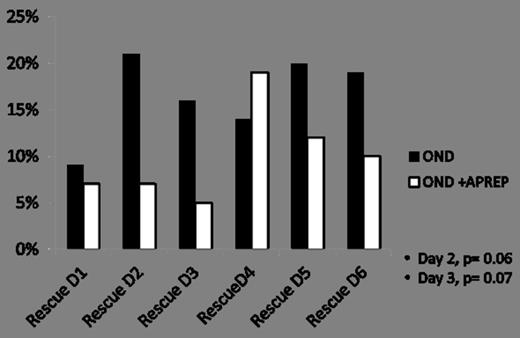
Forty nine pts were enrolled in each arm. Fifty seven percent were men in arm 1 and 55% men in arm 2. The median age was 53yrs (ranges, 30-68) in arm 1 and 49yrs (ranges, 21-70) arm 2. Caucasians were 83% in arm 1 and 76% in arm 2. African American and Hispanics were 10 % and 6% respectively in arm 1 and 8% and 12% in arm 2. Among the population studied 96% were diagnosed with AML in each arm. MDS 2% in arm 1 and 4% in arm 2. Fourteen percent received Idarubicin with cytarabine (Ara-C) in each arm. Twenty percent in ondansetron (OND) arm and 16% in ondansetron plus aprepitant (OND+APREP) arm receive Ara-C combined with Fludarabin. Ara-C in combination with anthracycline plus nucleoside analogues was given to 49% of pts in each arm. Ten percent in arm 1 received Ara-C with anthracycline and targeted therapy, 8% in arm 2 receive similar combination. Forty two pts on OND arm and 41 pts in the OND+APREP arm were evaluable for efficacy. Patient in the OND+APREP arms achieved higher overall response rates (complete plus partial responses) as shown in Figure 1, but the difference was not statistically significant (OND 67%, OND+APREP 80%; P= 0.11). More than 75% of pts overall were free of nausea on Days 1 and 2. On days 6 and 7 higher proportion of pts in OND+APREP arm were free from nausea than OND arm. Overall 36% of pts required rescue medications, 38% in OND arm and 34% in OND+APREP arm. Among them 14% of pts required rescue more than once; 19% in the OND arm and 10% in OND+APREP arm, as shown in Figure 2. Requirement of rescue medications on Days 2 and 3 were fewer in OND+APREP arm 7% and 5% respectively compare to 21% and 16% in the ondansetron arm (P = 0.06 and P=0.07).
Most common adverse events were headache and diarrhea. Twenty percent of pts in OND arm and 19% in OND+APREP arm reported to have NCI grade 2 headache (P= 0.56). Five percent of pts in ondansetron arm and 12% in ondansetron plus aprepitant arm had grade 2 diarrhea (P=0.19).
The daily assessment of emesis and nausea did not show statistically significant differences between the study arms but there was a clear trend towards better responses in ondansetron plus aprepitant arm with more overall response rates and lower use of rescue medications. Further larger studies are warranted to further define the benefits of this combination of antiemetic drugs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal