Abstract
Mutations in DNMT3A (encoding one of two mammalian de novo DNA methyltransferases) are found in >30% of normal karyotype AML cases and correlate with poor clinical outcomes. Most DNMT3A mutations occur at position R882 within the catalytic domain (most commonly R882H) and are virtually always heterozygous. This over-representation suggests that mutations at R882 may result in gain-of-function or dominant-negative activity that contributes to leukemogenesis. However, how DNA methylation might be altered in DNMT3A-mutant cases of AML remains unclear, and no published study to date has addressed the effects of mixing wild-type (WT) and R882H DNMT3A. Importantly, mouse HSPCs deficient in Dnmt3a dramatically expand over time and have a concurrent defect in differentiation (Challen, GA et al. Nat Genet, 2011). Mice haploinsufficient for Dnmt3a, on the other hand, do not have a measurable defect in hematopoiesis. Collectively, these data suggest that the heterozygous R882 mutations probably cause more than a simple loss-of-function phenotype.
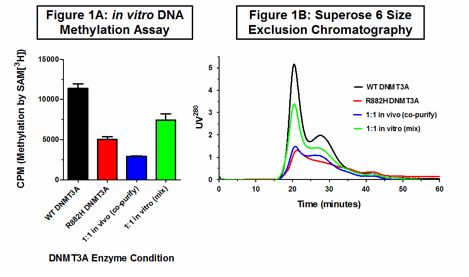
We purified full-length, human WT and R882H DNMT3A using a mammalian tissue culture system to produce recombinant proteins for biochemical modeling of the de novo methylation potential of a DNMT3A-mutant AML cell. rhR882H DNMT3A exhibits roughly 10-20% of the de novo DNA methyltransferase activity of rhWT DNMT3A, similar to observations by other groups. We added increasing amounts of R882H DNMT3A to a fixed amount of WT DNMT3A and observed a linear increase in the net enzymatic activity, reflecting the summed activity of the two forms of DNMT3A in these 4-hour in vitro reactions. In contrast, 12-hour in vitro DNA methylation assays with mixed WT and R882H DNMT3A demonstrated net methylation less than the predicted summed activity of the two enzymes, suggesting that a dominant-negative effect of R882H DNMT3A may occur with a long equilibration time.
To better simulate an AML cell with a heterozygous R882H mutation, we co-transfected HEK293T cells with equal amounts of poly-His-tagged WT and R882H DNMT3A expression vectors. Subsequently co-purified (i.e. in vivo-mixed) WT and R882H DNMT3A exhibited a striking reduction in methyltransferase activity, with total activity similar to R882H DNMT3A alone (Figure 1A). TSQ mass spectrometry allowed us to verify the presence and quantify the relative concentration of WT and R882H DNMT3A in our co-purified samples. We exploited a novel tryptic cleavage site in DNMT3A produced by the R882H mutation to generate standard concentration curves using recombinant peptides distinguishing the two protein forms. Our co-purified enzyme preparations had WT:R882H ratios ranging from 0.79 to 1.60; all demonstrated the dominant-negative effect of R882H.
DNMT3A is a processive enzyme, catalyzing multiple methyl-group transfers before dissociating from target DNA. This is dependent on the ability of WT DNMT3A to form homo-oligomers (tetramers and larger), which was recently shown to be disrupted by the R882H mutation using the catalytic domain of DNMT3A produced in E.coli (Holz-Schietinger, C et al. JBC, 2012). We therefore postulated that the dominant-negative effect of R882H may be due to the disruption of WT DNMT3A oligomerization. Using a Superose 6 size exclusion column, we confirmed the tetramerization defect of R882H DNMT3A relative to WT DNMT3A. Notably, in vivo-mixed (co-purified) WT and R882H DNMT3A complexes exhibited a pattern of oligomerization identical to R882H DNMT3A alone. However, WT and R882H DNMT3A mixed in vitro exhibited a distribution of oligomers corresponding to the expected average of the WT and R882H curves (Figure 1B).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal