Abstract
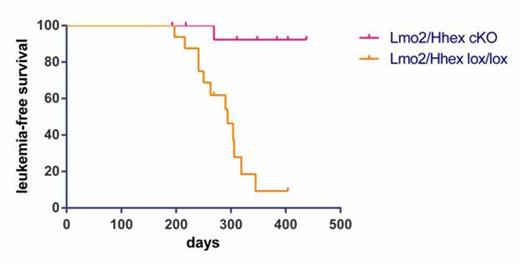
Hematopoietically expressed homeobox (Hhex) is a T-cell oncogene. It is frequently deregulated in murine retroviral insertional mutagenesis screens and its enforced expression induces T-cell leukemia in bone marrow transduction and transplantation experiments. We discovered that HHEX is a direct transcriptional target of an LIM domain Only-2 (LMO2)-associated protein complex. HHEX clusters with LMO2-overexpressing T-ALLs and is especially overexpressed in Early T-cell Precursor (ETP) – ALL where it is a direct transcriptional target of LMO2. To further understand Hhex's function, we induced a conditional knockout in floxed Hhex mice with the Vav-iCre transgene. Mice were viable and showed normal blood cell counts with highly efficient deletion of Hhex in all hematopoietic tissues. Thymocytes from conditional knockouts showed a normal pattern of development. Most impressively, Hhex conditional knockout markedly prolonged the latency of T-ALL onset in CD2-Lmo2 transgenic mice (figure 1). Hhex conditional knockouts (Hhex cKOs) also had a significant decrease in mature B cells in the spleen and bone marrow. Interestingly, hematopoietic stem and progenitor cells plated on OP9-GFP or OP9-DL1 stromal cells showed proliferative defects and incomplete differentiation towards both B and T lineage. Also under stress conditions such as sublethal irradiation and competitive bone marrow transplants, Hhex conditional knockouts show a marked defect in both B and T lineages but an increase in early progenitor populations. Our experiments show that Hhex is a critical transcription factor in lymphoid development and in LMO2-induced T-ALL. Close modal
Figure 1
Hhex conditional knockout markedly prolonged the latency of T-ALL onset in CD2-Lmo2 transgenic mice
Figure 1
Hhex conditional knockout markedly prolonged the latency of T-ALL onset in CD2-Lmo2 transgenic mice
Disclosures:
No relevant conflicts of interest to declare.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2013 by The American Society of Hematology
2013

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal