Abstract
Antibody dependent cellular cytotoxicity (ADCC) is one of the mechanisms by which therapeutic antibodies mediate tumor cell killing. The anti-CD20 antibody rituximab is the current standard of care in the treatment of B-cell lymphomas. GA101, a novel anti-CD20 antibody, contains a glycoengineered Fc-portion allowing approximately 10-fold greater affinity to FcgR3A, the Fc-IgG receptor expressed on the majority of natural killer (NK) cells.
NK cell function is also regulated by inhibitory killer-cell immunoglobulin-like receptors (KIR), which interact with HLA class I antigens (2DL1-HLA-C2; 2DL2/3-HLA-C1, 3DL1-HLA-Bw4). The KIR/HLA interaction during NK cell development leads to the acquisition of full effector function in the “licensing” process, but also provides one of the main mechanisms of NK cell tolerance. The present study analyzed how KIR/HLA interactions influence ADCC, and whether there are differences between conventional and glycoengineered antibodies. We analyzed the activation (in terms of the degranulation measured by the CD107a expression) and killing capacity of KIR-positive NK cells induced by rituximab, GA101, and the parental non-Fc modified (wild-type) GA101wt. Target cells included HLA-negative B-cell lymphoma lines or B-cell lines expressing one or more HLA molecules.
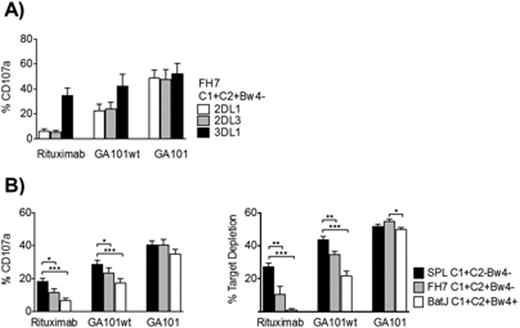
We confirmed previous observations that the licensing status affects the potential for rituximab-induced ADCC (degranulation against HLA-deficient 721.221 in licensed cells 35 ± 4% versus 19 ± 3% of unlicensed cells, p<0.01); and that KIR/HLA interactions strongly and selectively inhibit the response to targets expressing cognate HLA ligands (e.g. CD107a expression in KIR3DL1+ NK cells 17 ± 3% against 721.221-Bw4 cells, compared to 32 ± 4% against 721.221, p<0.01). Next, we analyzed rituximab-induced NK cell activation in donors expressing one, two, or three KIR ligands after co-incubation with target B-cell lines expressing corresponding HLA molecules. These experiments showed that the inhibitory effect during target cell encounter dominates over the activating effect of NK cell licensing, which leads to unlicensed NK cells being the strongest effectors of ADCC with rituximab (Figure, Panel A).
We next compared the effect of the KIR/HLA interaction on rituximab-, GA101wt- and GA101-induced ADCC. GA101 largely compensated the hyporesponsiveness of unlicensed cells and NK cell activation was independent of the presence of HLA KIR ligands on target cells (Figure, Panel A). Finally, we addressed the question of how levels of NK cell degranulation correspond to target cell elimination. Correlation between CD107a expression and target cell elimination was excellent for all antibodies (Figure Panel B). GA101 induced the highest level of activation and the most effective target elimination. In contrast to rituximab and GA101wt, no negative impact of KIR/HLA interaction on degranulation or target cell elimination could be detected for GA101.
In summary, we show that KIR/HLA interactions are relevant for ADCC with rituximab, with the negative impact during target cell encounter dominating over the positive effect of licensing. In contrast, the novel glycoengineered GA101 antibody overrides the negative signals derived from the KIR/HLA interaction and activates all NK cell subsets. These data suggest that the Fc-modification to enhance ADCC can be an effective strategy to augment the efficacy of therapeutic monoclonal antibodies by recruiting NK cells irrespective of their inhibitory KIR expression.
Terszowski:Roche: Research Funding. Klein:Roche Glycart AG: Employment. Stern:Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal